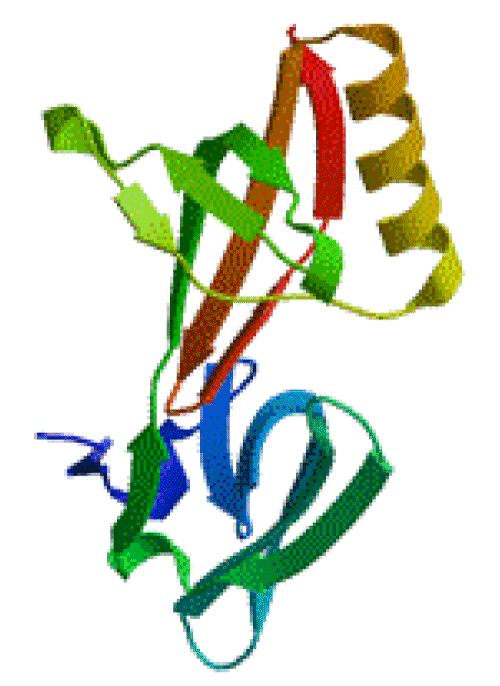Abstract
Panax ginseng is a commonly used herb in traditional Chinese medicine with a wide range of valuable medicinal usages. Modern pharmacology studies reveal that the Panax ginseng has great immune enhancement function. Translation initiation factor eIF-5a is one of its active proteins which have important function. In this paper, several physical and chemical fundamental characteristics such as isoelectric point and the hydrophobicity, etc, of translation initiation factor eIF-5a are studied. The molecular weight of translational initiation factor eIF-5a in Panax ginseng is 17221.44, its theoretical isoelectric point is 5.46, the instability index of translational initiation factor eIF-5a is 29.93, it could be concluded that its structure is stable from the results. There is none transmembrane regions and signal peptide cleavage sites in amino acid sequences of the translation initiation factor eIF-5a. Then, the secondary structure, 3D structure and phosphorylation sites of translation initiation factor eIF-5a are predicted. The results show that the number of potential phosphorylation sites of translation initiation factor eIF-5a is 19 (potential value > 0.5). All these result benefit for further study on function and usage of translation initiation factor eIF-5a plant.
Key words
translation initiation factor 5a (eIF-5a), Panax ginseng, bioinformatics analysis
Introduction
In traditional Chinese medicine, Panax ginseng is a valuable herbal medicine, which has been applied to resist various diseases, contains saponins, sucrose, polysaccharides, amino acids, and so on. Panax ginseng has many good effects for health, such as anti-haemostatic [1], anti-tumor [2], antiaging [3], enhancing immunity [4] and antioxidative effect [5], etc; all of these effects can be used to treat disease. Many functions of Panax ginseng are explored by lots of scholars. Cesare Mancuso, et al. discussed the pharmacology and toxicology of Panax ginseng, they tent to think that Panax ginseng is benefit to central nervous system, metabolic, infectious and neoplastic diseases [6]. Chia-Rou Yeo, et al. studied the isolation and characterization methods of bioactive polyacetylenes Panax ginseng [7]. Byong-Kyu Shin, et al. explored the chemical diversity of saponins from Panax ginseng, they recapitulated the chemical structures and monoisotopic masses of saponins from Panax ginseng [8]. Ying Zhou, et al. studied the changes in element accumulation and antioxidative enzyme activities in Panax ginseng, the authors attend to think that the higher element accumulation activated multiple enzymes related to accumulation of phenolic compounds and their oxidation [9]. Panax ginseng in medicine has many effects on human health [10], and it is also used to treat many diseases [11], such as obesity and adipose inflammation [12], chronic obstructive pulmonary disease [13]. In bioinformatics, there are also lots of scientists studied these issues [14], Yun Sun Lee, et al. explored the transcriptomes and primary metabolite profiles of different Panax ginseng cultivars [15], Ming-Rui Li, et al. studied the genetic and epigenetic diversities of Panax ginseng [16]. But when the translation initiation factor eIF-5a is concerned, it plays a variety of functions in plant, may effect on cell senescence and death, so the translation initiation factor eIF-5a attracts many scholars [17]. In this paper, main physical characteristic of translation initiation factor eIF-5a in Panax ginseng is studied from the bioinformatics view.
Materials and methods
In order to, study the translation initiation factor eIF-5a in Panax ginseng, the peptide protein sequences data is download from NCBI (accession number: AIC33055.1). The fundamental characteristics of its sequence and the functional results are respectively studied in this paper.
Fundamental characteristics are studied via the protparam system and protscale system [18], TMHMM online system [19], et al. to explore its physical and chemical properties. Then the SignalP system [20], SOPMA online system [21], SWISS-MODEL system [22] and the online system NetPhos [23], et al. are used to study its Physiological functions. All prediction data are re-plotting for their aesthetic requirements.
Results and discussion
Physical and chemical properties of the translation initiation factor eIF-5a is studied by protparam system, protscale system, TMHMM online system, et al. partial fundamental properties is shown in Table 1. that is number of amino acids is 159, molecular weight is 17221.44, instability index is 29.93, the negatively residues and positively residues respectively are 25 and 19. TMHMM predicted system is used to predict the transmembrane region of translation initiation factor eIF-5a and the result show that there is no transmembrane region in translation initiation factor eIF-5a.
Table 1. Fundamental properties of translation initiation factor eIF-5a.
Parameter |
Perdition results |
Number of amino acids |
159 |
Molecular weight |
17221.44 |
Theoretical pI |
5.46 |
Formula |
C749H1202N206O244S7
|
Total number of atoms |
2408 |
Instability index |
29.93 |
Negatively residues (Asp + Glu) |
25 |
Positively residues (Arg + Lys) |
19 |
Hydrophobic and hydrophilic in translation initiation factor eIF-5a appears alternately. Hydrophobicity of translation initiation factor eIF-5a is predicted by the Hphoh./Kyte & Doolittle scale in ExPASy's ProtScale program with window size = 9. The prediction result is shown in Figure 1, when the vertical value > 0 denotes the hydrophobic area, less than zero is the hydrophilic area. There is an opposite strong Hydrophobicity can be seen in translation initiation factor eIF-5a. It can be seen that there are more hydrophilic residues than the hydrophobic residues in general. Among the whole residues, only 2 absolutely value of residues area are more than 2.
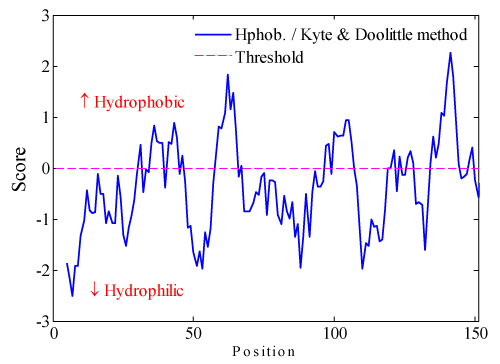
Figure 1. Hydrophobic characteristics of translation initiation factor eIF-5a.
When the function of the translation initiation factor eIF-5a is concerned, SignalP online prediction system is used to predict whether there is any signal peptide in its sequence. The prediction result shows that there is no signal peptide sequence in the translation initiation factor eIF-5a. Then the secondary structure of the translation initiation factor eIF-5a is predicted via the SOPMA online system with the parameters: Window width=17, Similarity threshold=8, and the Number of states=4(Helix: 0.5970, Sheet: 0.9710, Turn: 1.5730, and Coil: 0.7740). The result is shown in Figure 2. The result shows that in its secondary state, four rates for each state are: Alpha helix: 25.79%, Beta sheet: 20.75%, Turn: 14.47%, and Coil: 38.99%.
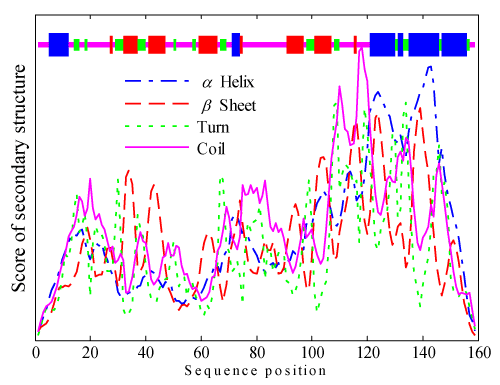
Figure 2. Secondary structure of translation initiation factor eIF-5a.
The famous Swiss-Model system is usually used to predict the 3D structure of a protein. The 3D structure of the translation initiation factor eIF-5a here is predicted by the online Swiss-Model system, and the result is shown in Figure 3. From Figures 2 & 3, it could be conclude that the translation initiation factor eIF-5a contains variety of secondary structural units with obvious folding levels, furthermore, the translation initiation factor eIF-5a presents a ellipsoidal entity shape.
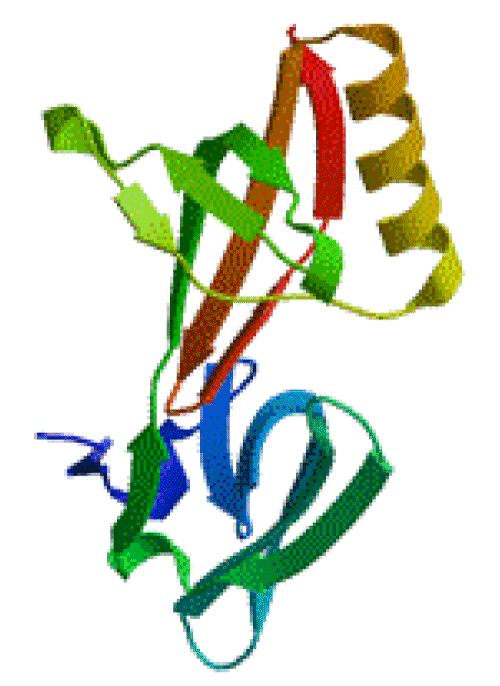
Figure 3. 3D structure of translation initiation factor eIF-5a.
Quantitative studies of protein phosphorylation are benefit to the study on their biological activities. The NetPhos protein phosphorylation site is used to predict the phosphorylation sites of the translation initiation factor eIF-5a. The result is shown in Figure 4. The value of serine phosphorylation sites is shown to be high, and there are two high potential Serine phosphorylation sites, three high potential Threonine phosphorylation sites and only one high potential Tyrosine phosphorylation site within the prediction results (potential value>0.8). All of the shown phosphorylation sites are potential value >0.5 and the other phosphorylation sites (potential value <0.5) are neglected.
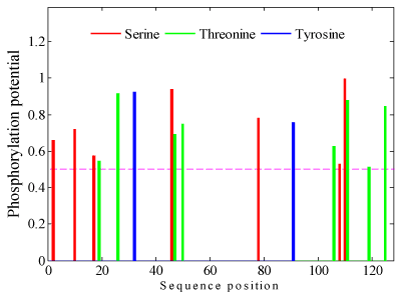
Figure 4. Phosphorylation site prediction results of the translation initiation factor eIF-5a.
Recent studies have found that translation initiation factor eIF-5a is closely related to the metabolism of polyamines and the occurrence of tumors, which is one of the hot research areas of concern in recent years. Application of Panax ginseng in traditional medicine for antitumor in China has been many years [24]. Lots of scientists have been studied its bio-functions by from different aspect, such as its antitumor and immunomodulatory activity [25], antioxidant activity [26], analgesic [27], anticomplement [28], and so on [29], but the specific mechanism of medicinal ingredients of Panax ginseng such as the translation initiation factor eIF-5a is still need further study especially from the perspective of bioinformatics [30].
Conclusion
Translation initiation factor eIF-5a is prevalent in eukaryotic and prokaryotic organisms. It is a highly conserved protein whose C-terminus is a nucleic acid binding domain, and N-terminus is highly conserved and contains a very unique amino acid. Translation initiation factor eIF-5a plays an important role in the protein translation extension phase by stimulating the activity of the protein involved in the ribosome. The molecular mechanism of the translation initiation factor eIF-5a has not yet been fully elucidated, and further research is needed to deepen the understanding of its function. In this study, the biological information especially the physical and chemical characters contained in translation initiation factor eIF-5a are systematically studied from its sequence, its other properties are predicted via several famous bioinformatics tools, which provided a reference for further research on Panax ginseng.
Acknowledgments
This research work was supported by president fund of Xi’an Technological University (No.XAGDXJJ14011).
References
- Fung FY, Wong WH, Ang SK, Koh HL, Kun MC, et al. (2017) A randomized, double-blind, placebo- controlled study on the anti-haemostatic effects of Curcuma longa, Angelica sinensis and Panax ginseng. Phytomedicine 32: 88-96. [Crossref]
- Castro-Aceituno V, Ahn S, Simu SY, Singh P, Mathiyalagan R, et al. (2016) Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed Pharmacother 84: 158-165. [Crossref]
- Hwang E, Park SY, Yin CS, Kim HT, Kim YM, et al. (2017) Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J Ginseng Res 41: 69-77. [Crossref]
- Kim HG, Jang SS, Lee JS, Kim HS, Son CG (2017) Panax ginseng Meyer prevents radiation-induced liver injury via modulation of oxidative stress and apoptosis. J Ginseng Res 41: 159-168. [Crossref]
- Chung IM, Lim JJ, Ahn MS, Jeong HN, An TJ, et al. (2016) Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J Ginseng Res 40: 68-75. [Crossref]
- Mancuso C, Santangelo R (2017) Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem Toxicol 107: 362-372. [Crossref]
- Yeo CR, Yong JJ, Popovich DG (2017) Isolation and characterization of bioactive polyacetylenes Panax ginseng Meyer roots. J Pharm Biomed Anal 139: 148-155. [Crossref]
- Shin BK, Kwon SW, Park JH (2015) Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res 39: 287-298. [Crossref]
- Zhou Y, Yang Z, Gao L, Liu W, Liu R, et al. (2017) Changes in element accumulation, phenolic metabolism, and antioxidative enzyme activities in the red-skin roots of Panax ginseng. J Ginseng Res 41: 307-315. [Crossref]
2021 Copyright OAT. All rights reserv
- Gray SL, Lackey BR, Boone WR (2016) Effects of Panax ginseng, zearalenol, and estradiol on sperm function. J Ginseng Res 40: 251-259. [Crossref]
- Colzani M, Altomare A, Caliendo M, Aldini G, Righetti PG, Fasoli E (2016) The secrets of oriental panacea: Panax ginseng. J Proteomics 130: 150-159. [Crossref]
- Lee H, Choi J, Shin SS, Yoon M (2016) Effects of Korean red ginseng (Panax ginseng) on obesity and adipose inflammation in ovariectomized mice. J Ethnopharmacol 178: 229-237. [Crossref]
- Shergis JL, Di YM, Zhang AL, Vlahos R, Helliwell R, et al. (2014) Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement Ther Med 22: 944-953. [Crossref]
- Khorolragchaa A, Kim YJ, Rahimi S, Sukweenadhi J, Jang MG, Yang DC (2014) Grouping and characterization of putative glycosyltransferase genes from Panax ginseng Meyer. Gene 536: 186-192. [Crossref]
- Lee YS, Park HS, Lee DK, Jayakodi M, Kim NH, et al. (2017) Comparative analysis of the transcriptomes and primary metabolite profiles of adventitious roots of five Panax ginseng cultivars. J Ginseng Res 41: 60-68. [Crossref]
- Li MR, Shi FX, Zhou YX, Li YL, Wang XF, et al. (2015) Genetic and epigenetic diversities shed light on domestication of cultivated ginseng (Panax ginseng). Mol Plant 8: 1612-1622. [Crossref]
- Liu J, Wang Q, Sun M, Zhu L, Yang M, et al. (2014) Selection of reference genes for quantitative real-time PCR normalization in Panax ginseng at different stages of growth and in different organs. PLoS One 9: e112177. [Crossref]
- Gasteiger E, Hoogland C, Gattiker A, et al. (2005) Protein Identification and Analysis Tools on the ExPASy Server, Proteomic Protocols Handbook, 112: 571-607.
- Xie Yong, Hong Xiaokun, Yan Renxiang, et al. Bioinformatics analysis of the recombinant rAgaN3 gene of agarose. Chinese Journal of Bioinformatics 15: 16-26.
- Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8: 785-786. [Crossref]
- Geourjon C, Deléage G (1995) SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci 11: 681-684. [Crossref]
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, et al. (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42: W252-258. [Crossref]
- Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S (2004) Kinase specific predictions: Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4: 1633-1649. [Crossref]
- Lili Jiao, Xiaoyu Zhang, Bo Li, et al. (2014) Anti-tumour and immunomodulatory activities of oligosaccharides isolated from Panax ginseng C.A. Meyer. Int J Biol Macromol 65: 229-233. [Crossref]
- Silvestrini P, Beccaria C, Pereyra EAL, Renna MS, Ortega HH, et al. Intramammary inoculation of Panax ginseng plays an immunoprotective role in Staphylococcus aureus infection in a murine model. Res Vet Sci 115: 211-220. [Crossref]
- Chung IM, Siddiqui NA, Kim SH, Nagella P, Khan AA, et al. (2017) New constituents triterpene ester and sugar derivatives from Panax ginseng Meyer and their evaluation of antioxidant activities. Saudi Pharm J 25: 801-812. [Crossref]
- Wang Y, Chen Y, Xu H, Luo H, Jiang R (2013) Analgesic effects of glycoproteins from Panax ginseng root in mice. J Ethnopharmacol 148: 946-950. [Crossref]
- Hong-wei Gao, Miao-miao Zhang, Yan-li Liu, et al. (2013) Anticomplement activity of ginsenosides from Panax ginseng. J Functional Foods 5: 498-502.
- Patel S, Rauf A (2017) Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed Pharmacother 85: 120-127. [Crossref]
- Lu C, Zhao S, Wei G, Zhao H, Qu Q (2017) Functional regulation of ginsenoside biosynthesis by RNA interferences of a UDP-glycosyltransferase gene in Panax ginseng and Panax quinquefolius. Plant Physiol Biochem 111: 67-76. [Crossref]