According to recent bibliography,prenatal diagnosis reflects meticulous depiction of imaging findings in order to establish proper therapeutic mapping of potential congenital malformations. Predisposition factors such as age of the mother, absence or hypoplasia of nasal bone, increased NT (nuchal translucency) measurement, heredity, gene disorders are strongly accompanied with congenital deviations. Sonographic imaging findings of fetal facial anatomic area can provide information concerning antenatal diagnosis of fetuses with various congenital syndromes and chromosomal aberrations. Deviation from normal proportions of fetal facial profile not only might consist of ‘soft sonographic signs’, leading to important clues, but also suggests presence of chromosomal disorders. Binder Syndrome is characterized by midfacial hypoplasia, associated with absence of nasal spine, leading to a flat profile and depressed nasal bridge. Aim of our study consists prenatal presentation of rare case series of Binder Syndrome, proper diagnosed and assiduous treated.
binder syndrome, nasal spine, congenital malformations, facial deviation
Meticulous prenatal diagnosis reflects proper gestation surveillance and confrontation of major congenital abnormalities. Many genetic or chromosomal disorders lead to severe fetal malformations and in most of cases in termination of gestation. Binder Syndrome is considered to be a heterogeneous phenotype, rather than a single nosologic entity [1].
On the other hand, pathogenic etiology remains a controversial issue. Ultimate scope consists assiduous prenatal diagnosis and treatment.
As multiphenotypic entity, Binder Syndrome depicts midfacial hypoplasia, absence of nasal spine, creating a ‘flat profile ‘and depressed nasal bridge [2]. Short nasal and columella formation, flat nasolabial angle and perialar flattering are strongly accompanied with phenotypic formation of Binder Syndrome [3].
Many conducted studies suggest autosomal dominant route as pathway of phenotypic transmission followed by increased percentage of questionable [4]. Majority of cases are sporadic. Nevertheless, many authors reported also familial occurrence [5]. In such cases, phenotypic transmission can be autosomal dominant, or associated with multifactorial inheritance.
Focusing on differential diagnosis of Binder Syndrome, exclusion of similar abnormalities with chromosomal origin is mandatory [6]. Binder Syndrome can be associated with Trisomy 21 or Down Syndrome. In order to establish proper diagnosis, karyotype evaluation seems to be mandatory.
However, 4p deletion must be eliminated. (Wolf-Hirschhorn Syndrome). Prenatal diagnosis of congenital abnormalities with genetic or chromosomal etiology consists gold standard during pregnancy. Therapeutic mapping depends on medical expertise and patient compliance. Presentation of our case series is strictly associated with assiduous prenatal and potential therapeutic strategy.
We present three cases of Binder Syndrome, characterized by leveling of frontal angle, meticulous prenatal diagnosed in the Department of Prenatal Diagnosis, University of Thessaly. Written consent underwent by the patients, in order to depict following manuscript.
Case 1
Patient 16-year-old P1G0with negative atomic history, without use of pharmaceutical agents or alcohol abuse.
Performance NT (nuchal translucency) (12w) and B level scanning (21w3d). NT and CRL (crown rump length) measurement 1,5mm and 62mm respectively. Hazard ratio for Trisomy 21 or Down Syndrome1/8500.
During B level scanning phenotypic formation of Binder Syndrome was diagnosed, followed by leveling of frontal angle and nasal bone hypoplasia. No signs of hypertelorism, micrognathia or muscle dysplasia (Figures 1a and 1b).
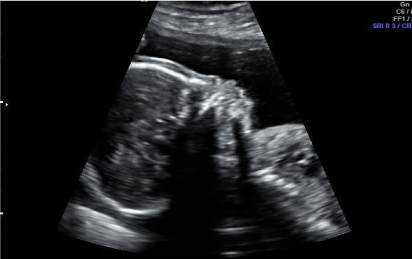
Figure 1a. Phenotypic depiction of Binder Syndrome with nasal bone hypoplasia and leveling of frontal angle
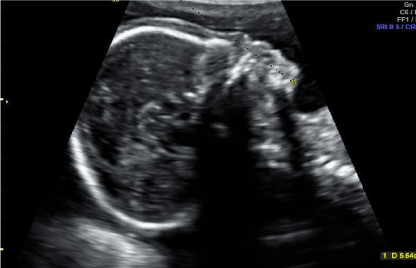
Figure 1b. Phenotypic depiction of Binder Syndrome with nasal bone hypoplasia and leveling of frontal angle
Due to nasal bone hypoplasia, in order to differential diagnose Trisomy 21 or Down Syndrome, amniocentesis was performed. Karyotype evaluation revealed chromosomal normal range (46 XY). Molecular karyotype was performed in order to differential diagnose Wolf Hirschhorn Syndrome. (4p deletion)
Sonographic evaluation (27w) confirmed all prenatal imaging findings. Amnion membranes were ruptured automatically (40w), followed by natural birth of one male fetus (3300g) without further operative complications.
Phenotypic evaluation revealed leveling of frontal angle and hypoplasia of distal phalanges of higher extremities. Fetus was discharged at 4th pod in good clinical condition.
Case 2
Patient 26-year-old P2G1 with history of one natural birth, underwent NT (nuchal translucency) (13w) and B level scanning (22w2d) respectively. Atomic history of hyperemesis gravidarum with use of pharmaceutical agents during first trimester of gestation. NT (nuchal translucency) and CRL (crown rump length) 1,7mm and 68 mm respectively. Hazard ratio for Trisomy 21 or Down Syndrome 1/6440. During B level scanning. Normal nasal bone and leveling of frontal angle were diagnosed (Figure 2). No signs of hypertelorism, micrognathia or muscle dysplasia.
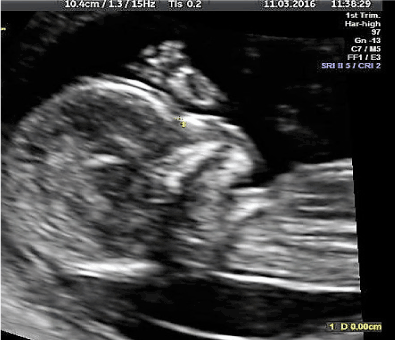
Figure 2. Leveling of fetal profile (12w. Binder Syndrome)
Fetal karyotype evaluation was suggested in order to differential diagnose potential chromosomal or genetic abnormalities. Both parents decided completion of gestation without prenatal invasive procedures. Sonographic evaluation(32w) confirmed all preoperative imaging findings. Patient underwent caesarian section (40 w) with birth of one female fetus(3040g). Fetal phenotypic evaluation revealed leveling of nasal bone and frontal angle.
Neonatal examination suggests potential development of Binder Syndrome. Fetus was discharged at 4th pod in good clinical condition.
Case 3
Patient 23-year-old G1P1 with history of hyperemesis gravidarum with use of pharmaceutical agents during first trimester of gestation. NT (nuchal translucency) (12w) and B level scanning (22w4d) were performed. NT (nuchal translucency) and CRL (crown rump length) measurement 1,5mm and 60 mm respectively. Hazard ratio for Trisomy 21 or Down Syndrome 1/8454. During B level scanning normal nasal bone and leveling of frontal angle were diagnosed (Figure 3).
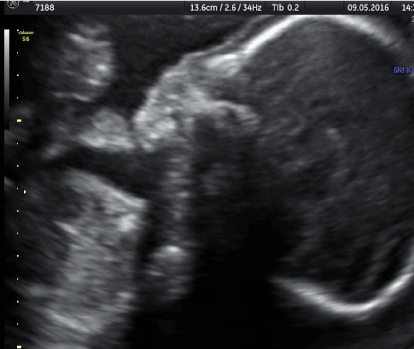
Figure 3. Normal nasal bone formation,leveling of frontal angle.(Binder Syndrome. B’ level scanning)
Fetal karyotype evaluation was suggested in order to differential diagnose potential chromosomal or genetic abnormalities. Both parents decided completion of gestation without prenatal invasive procedures. Potential differential diagnosis development of Binder Syndrome without exclusion of other chromosomal or genetic malformations.
Patient underwent natural birth (39w) with one male fetus (2980g). Fetal phenotypic evaluation revealed leveling of nasal bone and frontal angle. Neonatal examination suggests potential development of Binder Syndrome.Fetus was discharged at 4th pod in good clinical condition.
Sonographic evaluation during B’ level scanning consists gold standard in proper differential diagnosis, excluding or detecting clinical entities such as hypoplasia of nasal bone, micrognathia or leveling of fetal profile (Table 1).
Table 1. Facial abnormalities, sonographic imaging findings, correlation with clinical syndromes
Syndromes |
Sonographic facial imaging findings |
Other sonographic imaging findings |
Heredity |
Karyotype |
Wolf-Hirschhorn |
Leveling frontal angle |
Facial area like ancient Greek helmet |
None |
Normal |
Down |
Short nasal bone, prenasal edema |
Short brachial and femoral bone, hypoplasia 3rd phalanx small hand finger |
None |
Trisomy 21 |
Keutel |
Leveling frontal angle |
Short bones, hypoplasia 1st phalanx |
Autosomal recessive |
Normal |
Chondrodysplasia punctata |
Nasal hypoplasia |
Asymmetric shortage of long bones, Scoliosis |
X-linked dominant |
Normal |
Apert |
Nasal leveling, maxillary hypoplasia, hypertelorism |
Craniosynthesis, |
Autosomal dominant |
Normal |
Crouzon |
Maxillary hypoplasia, hypertelorism |
Craniosynthesis, Ventriculomegaly,
Syndactyly |
Autosomal dominant |
Normal |
Achondroplasia |
Facial middle third hypoplasia with absence of nasal spine |
Large skull, protruding frontal bone, short bones |
Autosomal dominant |
Normal |
Rubinger |
Leveling nasal spine |
Short fingers |
Autosomal recessive |
Normal |
Warfarin use syndrome |
Leveling frontal angle |
Punctuated epiphyseal hypoplasia |
Teratogen |
Normal |
Robinow |
Leveling frontal angle, hypertelorism |
Short forearm, clinodactyly, macrocephaly |
Autosomal dominant |
Normal |
Stickler |
Cleft, Leveling frontal angle, micrognathia |
Osteochondrodysplasia |
Autosomal dominant |
Normal |
Binder et al. [7] first described clinical entity characterized with small nasal bone, leveling of frontal angle, leveling of nasal funnels and curved upper lip shape.
Phenotypic deviation of Binder Syndrome is strongly accompanied with variety of etiologic factors, not focusing on an individual clinical entity.
Vitamin K deficit in early embryogenesis (especially between 5 and 9 weeks) must be strictly eliminated. Inherited or extrinsic factors disrupting maternal Vitamin K metabolism are known to result in branchytelephangic Chondrodysplasia punctata with Binder Syndrome [8].
Chondrodysplasia punctata, X-linked recessive, branchytelephangic type consists the main phenotype of Binder Syndrome. Sheffield et al. [9] reported 103 cases of chondrodysplasia punctata during in a 20-year examination period. They suggested that Binder Syndrome represents a mild form of Chondrodysplasia punctate. According to recent studies, women affected with Systemic Lupus Erythematosus are associated with Binder phenotype characterized by stippled epiphyses, mimicking fetal Warfarin Syndrome [10].
Many authors related Vitamin K deficiency to the presence of circulating anticoagulant or antiphospholipid antibodies [11]. Quarrel et al. [12], suggested that more appropriate seems to be the use of phenotype Binder. Olow-Nordenram et al. and Gross-Kieselstein et al. [13] reported 2 cases of Binder phenotype between mother and daughter, implying heredity origin.
Keutel Syndrome described midfacial hypoplasia, characteristic clinical sign of Binder Syndrome [14]. Munroe et al. suggested that Binder Syndrome could be variation of Keutel Syndrome, expressing genetic mutation, coding cellular protein Gla [15]. Valentin et al. [16] proposed as autosomal recessive heredity form followed by decreased penetration, reappearance of Binder Syndrome among relatives.
In our case series, meticulous diagnosis of phenotypic profile confirmed postpartum, without any further neonatal indication. In terms of isolated cases, final prognosis is very good. Plastic or orthodontic intervention seems to be mandatory in order to ensure adequate chewing [17].
Many cases reported, incidence 5% of Binder Syndrome with congenital heart disease and congenital hearing loss [18]. Use of Vitamin K supplements are mandatory with ultimate scope the elimination of Binder Syndrome and hyperemesis gravidarum during first trimester of gestation.
Binder Syndrome represents a controversial entity with variety of phenotypic abnormalities. Sonographic evaluation during pregnancy depicts proper differential diagnosis and therapeutic strategy. Multidisciplinary approach is mandatory in order to establish meticulous treatment. Further studies must be conducted, achieving this ultimate scope.
Financial disclosure
The authors declared that this study
has received no financial support.
Conflict of interest
Author 1, Sotiriou S. declare that he has no conflict of interest.
Author 2, Sofoudis C. declare that he has no conflict of interest.
Author 3, Garas A. declare that he has no conflict of interest.
Author 4, Koukoura O. declare that she has no conflict of interest.
Author 5, Skentou H. declare that she has no conflict of interest.
Author 6, Satra M. declare that she has no conflict of interest.
Author 7, Daponte A. declare that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all patients who were included in our study.
Author contribution
- Sotirios Sotiriou: Protocol/project development, Data collection, Data analysis, Manuscript writing/editing
- Chrisostomos Sofoudis: Manuscript writing/editing
- Antonios Garas: Data collection, Manuscript writing/editing
- Ourania Koukoura: Data collection
- Hara Skentou: Data collection
- Satra Maria: Manuscript writing/editing
- Alexandros Daponte: Protocol/project development
- Binder KH (1962) Dysostosis maxilla-nasalis, ein archinencephaler Missbildungskomplex. Deutch Zahnaerztl Z 17: 438
- Arnaud E (2014) Some features of the nose in craniofacial malformations. Ann Chir Plast Esthet 59: 585-591. [Crossref]
- Arroyo HH, Olivetti IP, Santos VG, Weber R, Jurado JR (2015) Nasal reconstruction in Binder Syndrome. Br J Otorhinolaryngol 83: 488-489. [Crossref]
- Gross-Kieselstein E, Har-Even Y, Navon P, Branski D (1986) Familial variant of maxilllonasal dysplasia? J Craniofac Genet Dev Biol 6: 331-334. [Crossref]
- Mazzone E, Cos Sanchez T, Persico N, Cannie MM, Jani J (2019) Binder syndrome: a phenotype rather than a definitive diagnosis? Ultrasound Obstet Gynecol 53: 131-132. [Crossref]
- Hunt JA, Hobar PC (2002) Common craniofacial anomalies: the facial dysostoses. Plast Reconstr Surg 110: 1714-1725. [Crossref]
- Cantarell SM, Auara LS, Perez SP, Juanos JL, Navarro FM, et al. (2016) Prenatal diagnosis of Binder’s syndrome: report of two cases. Clin Exp Obstet Gynecol 43: 301-305. [Crossref]
- Sheffield LJ, Halliday JL, Danks DM, Rogers JG, Poulos A, et al. (1989) Clinical, radiological and biochemical classification of chondrodysplasia punctata. Am J Hum Genet 45(suppl.): A64
- Sheffield LJ, Halliday JL, Jensen F (1991) Maxilllonasaldysplasia (Binder’s syndrome) and chondrodysplasia punctata. J Med Genet 28: 503-504. [Crossref]
- Howe AM, Webster WS (1992) The warfarin embryopathy: a rat model showing maxillonasal hypoplasia and other skeletal disturbances. Teratology 46: 379-390. [Crossref]
- Howe AM, Webster WS, Lipson AH, Halliday JL, Sheffield LJ (1992) Binder's syndrome due to prenatal vitamin K deficiency: a theory of pathogenesis. Aust Dent J 37: 453-460. [Crossref]
- Quarrell OW, Koch Hughes HE. Maxillonasal dysplasia (Binder's syndrome) (1990) J Med Genet 27: 384-387. [Crossref]
- Roy-Doray B, Gradual A, Alembik, Stoll C (1997) Binder syndrome in a mother and her son. Genet Counsel 8: 227-233. [Crossref]
- Hur DJ, Raymond GV, Kahler SG, Riegert-Johnson DL, Cohen BA, et al. A novel MGP mutation in a consanguineous family: Review of the clinical and molecular characteristics of Keutel syndrome. Am J Med Genet A 135: 36-40. [Crossref]
- Munroe PB, Olgunturk RO, Fryns JP, Van Maldergem L, Ziereisen F, et al. (1999) Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nature Genet 21: 142-144. [Crossref]
- Olow-Nordenram M, Valentin J (1988) An etiologic study of maxilla-nasal dysplasia: Binder’s syndrome. Cad J Dent Res 96: 69-74. [Crossref]
- Drozdowski PH, Łątkowski I, Zachara MG, Wójcicki P (2017) Binder syndrome: Clinical findings and surgical treatment of 18 patients at the Department of Plastic Surgery in Polanica Zdrój. Adv Clin Exp Med 26: 427-437. [Crossref]
- Mudgrade DK, Patel JS, Nasiruddin M, Motghare PC (2014) Binder’s Syndrome: report of two cases. J Indian Acad Oral Med Radiol 26: 196-199. [Crossref]




