Abstract
Title: Beyond the means of natural selection: A case of end stage heart failure and advanced esophageal cancer successfully treated with an implantable left ventricular assist device (LVAD) and aggressive chemoradiation therapy.
Background: End-stage heart failure and advanced cancer individually share poor prognoses. The combination of the two conditions in the same patient is grave. Advances in management for both maladies continue to evolve. As such, the field of cardio-oncology has emerged as an important subspecialty. A case of Stage D heart failure treated with an implantable LVAD in a patient with subsequently found to have metastatic esophageal cancer illustrates the multi-specialty and multi-modality approach to this complex problem.
Case: A 72-year-old man with end-stage heart failure underwent placement of an LVAD as Destination Therapy. Seven months postoperatively he was found to have advanced gastroesophageal carcinoma. He underwent combination chemo-radiation therapy and remains on a chemotherapy regimen more than three year since the cancer diagnosis and almost four years since the LVAD implant.
Conclusion: The combination of cardiovascular disease and cancer continues to be a problem as the population. As such, treatments of these maladies have evolved accordingly. Patients with implantable LVADs and cancer are beginning to appear more regularly as the number of LVADs implanted increase. It will be important for the clinician to understand the complex issues related to this unique patient population.
Introduction
Heart Failure continues to plague millions of citizens throughout the world, accounting for billions of dollars in health care expenditure in the United States annually [1]. Esophageal cancer, ranked sixth among all cancers in mortality, affects 450,000 people worldwide with an alarming rise in its incidence [2]. Individually, these two diseases portend a grave prognosis in their most advanced stages, with three-year survival being more the exception than the rule. No one knows the survival rates for NYHA IV heart failure combined with Stage IV esophageal adenocarcinoma. However, it is safe to predict that survival beyond one year would be remarkable. The purpose of this case report is to describe a 72-year-old man who underwent placement of an implantable left ventricular assist device (LVAD) as destination therapy (DT) for Stage D heart failure. Postoperatively, he was found to have adenocarcinoma at the gastroesophageal (GE) junction with loco-regional metastases to lymph nodes as well as a malignant lytic lesion in the lumbar spine. He underwent chemoradiation therapy with longer survival (> 3 yrs) than what would have been predicted. The issues related to this case raise important questions related to this unique patient population.
Case
A 72-year-old man was admitted with acute decompensated heart failure, complaining of dyspnea, fatigue, and generalized weakness. His past medical history was significant for cardiac disease, including cardiomyopathy and atrial fibrillation, eventually requiring mitral valve replacement and concomitant coronary artery bypass grafting seven years prior to admission. Two years following the surgery, he required admission for cardiovascular symptoms at which time echocardiography demonstrated an ejection fraction of 15 to 20%. He was medically managed and subsequently underwent insertion of an implantable cardiac defibrillator (ICD). He was discharged and remained stable until this admission five years later.
On examination, there were signs of left and right heart dysfunction with pulmonary congestion as well as abdominal and lower extremity edema. Laboratory values were significant for hyponatremia (Na 129), renal dysfunction (BUN 50, Creat 2.1), and BNP elevation (2050). Echocardiography showed severe left ventricular dysfunction and moderate right ventricular dysfunction. Right heart catheterization showed elevated filling pressures with low cardiac output (CI 1.5 L/min/m2). Milrinone infusion was initiated and LVAD consideration was entertained. Following LVAD evaluation and consent, a Heartmate II (Thoratec, Inc., Pleasanton, CA) was implanted without complication. The hospital course was unremarkable and discharge to home took place on postoperative day sixteen.
During the first six months since the LVAD placement, the clinical condition improved to NYHA Class I. However, in the seventh postoperative month, the patient experienced melena requiring admission, evaluation, and red blood cell transfusion. Upper gastrointestinal (UGI) endoscopy was performed and showed a gastric mass with ulceration at the gastroesophageal junction. Biopsy of the lesion was positive for adenocarcinoma (Figure 1). Hematology-Oncology, General Surgery, and Radiation Therapy consults were obtained during the staging period. Full body computer tomography (CT), Upper Endoscopic Sonography (UES), and Positron Emission Tomography (PET) categorized the tumor as Stage III (T3N1). The maximal SUV of the lesion was 9.48. A multi-disciplinary discussion concluded that the course of treatment would be chemoradiation therapy. Surgical resection was not feasible in view of the LVAD position. A regimen of Carboplatin-Paclitaxel (TAXOL) for nine rounds followed by six weeks of radiation therapy was given. Approximately four months after the diagnosis, imaging of the lumbar spine with biopsy of an L4 lesion proved positive for metastatic poorly differentiated adenocarcinoma. Chemotherapy was continued with serial PET scans showing diminution and eventual disappearance of abnormal FDG uptake.
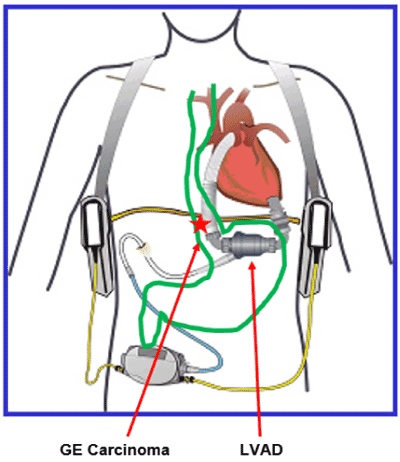
Figure 1. “Anatomy” of LVAD and Gastroesophageal carcinoma
Furthermore, follow-up UGI endoscopy showed normal benign gastric mucosa. During the treatment period, three complications occurred:
1) Portacath infection with Staphylococcus aureus requiring antibiotics, removal and replacement.
2) Bleeding following ICD removal and replacement requiring red blood cell transfusion and wound hematoma evacuation.
3) LVAD driveline site fracture requiring repair. At present (> 3 yrs), the patient remains on maintenance Oxaliplatin, leucovorin, fluorouracil (FOLFOX) therapy.
Discussion
Left Ventricular Assist Device (LVAD) placement for end-stage heart failure has increased in popularity for Destination Therapy (DT) since the landmark REMATCH Trial demonstrated superior efficacy of the first generation Heartmate (Heartmate I) over optimal medical therapy in 2001[3]. Improvements in LVAD technology has translated into more widespread application, with over 20,000 second generation Heartmate systems (Heartmate II) implanted and third generation units (Heartmate III) in trial [4]. As a result of this success, a growing body of experience has come to light with regard to the discovery of malignancies while on long-term LVAD support.
In a manuscript by Goldstein et al over twenty years ago, the authors described eight patients in whom non-cardiac surgery was performed with the Heartmate I [5]. While the majority of the cohort were operated on for benign disease, one patient required a nephrectomy for renal cell carcinoma. Resection for cancer in Heartmate II recipients and other implantable devices were reported by several authors in more recent publications [6-10]. In 2011, Murakawa et al described a left thoracotomy with lower lobectomy for a pulmonary nodule that was found to be an adenocarcinoma in a 58-year-old woman who underwent LVAD implantation as a BTT [6]. In 2014, Loyaga-Rendon et al analyzed 118 patients with continuous flow LVADs of which eight patients were found to have malignancies post-implant [7]. Two of the patients were diagnosed with localized esophageal adenocarcinoma. One of the patients (Stage T1N0M0) received radiofrequency ablation and subsequently died six months later of LVAD failure. The other patient (T3N0M0) received chemoradiation with Doxetacel and 5-FU—this patient expired fifteen months later. Other malignancies discovered in LVAD patients included malignant melanomas, basal cell carcinomas, Grade IV glioblastomas, multiple myelomas, small cell lung carcinoma, gastric carcinoma, renal cell carcinoma, and breast ductal cell carcinoma [6-10]. While the majority were diagnosed post-LVAD implant, a small number were discovered pre-implant during screening for transplantation [9] these were two cases of renal cell tumors and both were resected 8 and 9 months following LVAD implantation.
The case presented in this report is reflective of the state of affairs as it pertains to the two most common causes of mortality in Western societies cardiovascular disease and cancer. As a result of medical and technological advances, the treatment of these two conditions has radically changed (and improved), particularly as it relates to the most advanced stages of these maladies. Mechanical circulatory support with long-term implantable LVADs has demonstrated efficacy above and beyond medical therapy for patients with Stage D or NYHA Class IV heart failure, so much so that the indication for LVAD implantation has expanded from simply a BTT device to a DT device for patients ineligible for cardiac transplantation. As a result, more implantable LVADs have been placed annually than transplants performed since 2012 and, according to the Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs), 1379 of the 2919 implantable LVAD implants performed in calendar year 2015 were done for DT [11]. These trends will continue to grow and the consequences of them will be exposed cancer among them.
2021 Copyright OAT. All rights reserv
The issues of managing LVAD patients with cancer are multiple:
1) the possible need for surgery or interventional procedures in the setting of anticoagulation.
2) the effects of chemotherapy.
3) the effects of radiation therapy.
4) the psychological factors of living with two life-threatening conditions.
With regard to the need for surgical procedures and/or interventions to treat a cancer in an LVAD patient, the preoperative planning, intraoperative approach, and postoperative management are not trivial. Depending upon the nature and location of the procedure, particular care must be taken to avoid damage to or contamination of the LVAD. In addition, wound healing is important, particularly if there is a component of malnourishment in the patient related to the cancer or the heart failure. Furthermore, the management of anticoagulation is problematic: oral anticoagulation needs to be converted to IV anticoagulation with careful monitoring to maintain therapeutic levels during the perioperative period. The need for anticoagulation discontinuation needs to be considered if uncontrollable bleeding is encountered, subjecting the LVAD to possible thrombosis. With regard to chemotherapy and radiation therapy, there is a very limited understanding of the effects on the LVAD itself and the LVAD patient: this is a new area of investigation with important implications for planning adjuvant or neoadjuvant therapy in this unique patient population [12-13]. Finally, although some literature exists on the psychosocial and sexual issues in LVAD patients [14], there is no experience in the LVAD with cancer patient : another area for clinical research.
The barrier between malignancy and mechanical circulatory support whether as a BTT or DT , is breaking down. No longer is it an absolute contraindication to reject a patient for transplant or DT LVAD therapy because of cancer. In fact, this issue was addressed at the 2013 International Society for Heart and Lung Transplantation (ISHLT) in which the recommendation was made to consider patients with a history of a treated cancer who are in long-term remission or who are considered free of disease eligible for mechanical circulatory support as a BTT. Furthermore, patients with a history of recently treated or active cancer, who have a reasonable life expectancy (i.e. 2 years) may be candidates for DT [15]. The case described in this report adds to the growing list of LVAD patients with malignancy. It is illustrative of the ability to successfully treat life-threatening conditions with state-of-the-art therapies. Undoubtedly, more cancers will surface in patients whose lives were extended by mechanical circulatory support therapies. While the LVAD opens up new opportunities to treat some of these malignancies without the background or development of cardiac failure, the LVAD itself, by virtue of its ability to keep patients alive longer, will expose unexpected things, cancer among them. Charles Darwin, in his book On The Origin of Species by Means of Natural Selection, is quoted: “It is not the strongest of the species that survives, nor the most
intelligent, but the most responsive to change.” [16] Clearly, medicine and surgery together have changed the landscape of mankind’s ability to live beyond the means of natural selection.
References
- Voigt J, Sasha John M, Taylor A, Krucoff M, Reynolds MR, et al. (2014) A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol 37: 312-321. [Crossref]
- Zhang Y (2013) Epidemiology of esophageal cancer. World J Gastroenterol 19: 5598-5606. [Crossref]
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, et al. (2001) Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 345: 1435-1443. [Crossref]
- www.thoratec.com, www.heartmateii.com.
- Goldstein DJ, Mullis SL, Delphin ES, el-Amir N, Ashton RC Jr, et al. (1995) Noncardiac surgery in long-term implantable left ventricular assist-device recipients. Ann Surg 222: 203-207. [Crossref]
- Murakawa T, Murayama T, Nakajima J, Ono M (2011) Lung lobectomy in a patient with an implantable left ventricular assist device. Interact Cardiovasc Thorac Surg 13: 676-678. [Crossref]
- Loyaga-Rendon RY, Inampudi C, Tallaj JA, Acharya D, Pamboukian SV (2014) Cancer in end-stage heart failure patients supported by left ventricular assist devices. ASAIO J 60: 609-612. [Crossref]
- Maliha Khan, Anum Wasim, Aibek E. Mirrakhimov, Blaithin A. McMahon, Daniel P. Judge, Linda C. Chu, et al. (2015) Case Report of a Patient with Left Ventricular Assistance Device Undergoing Chemotherapy for a New Diagnosis of Lung Cancer. Case Reports in Oncological Medicine Volume, Article ID 163727, 3 pages.
- Smail H, Pfister C, Baste JM, Nafeh-Bizet C, Gay A, et al. (2015) A difficult decision: what should we do when malignant tumours are diagnosed in patients supported by left ventricular assist devices? Eur J Cardiothorac Surg 48: e30-36. [Crossref]
- Nakamura Y, Toda K, Nakamura T, Miyagawa S, Yoshikawa Y, et al. (2017) Curative surgery for gastric cancer in a patient with an implantable left ventricular assist device. J Artif Organs 20: 170-173. [Crossref]
- Intermacs Registry: www.uab.edu/medicine/intermacs/
- Gossman MS, Graham JD, Tamez D, Voskoboynikov N, Larose JA (2012) Evaluation of a ventricular assist device: stability under x-rays and therapeutic beam attenuation. ASAIO J 58: 212-216. [Crossref]
- Liane Y. Emerson, Matthew P. Deek, BA, Jesus Almendral, MD, Salma K. Jabbour (2016) Radiation therapy in patients with left ventricular assist device: A case report and literature review. Practical Radiation Oncology 6:e145-e147.
- Samuels LE, Holmes EC, Petrucci R (2004) Psychosocial and sexual concerns of patients with implantable left ventricular assist devices: a pilot study. J Thorac Cardiovasc Surg 127: 1432-1435. [Crossref]
- Feldman D1, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, et al. (2013) The 2013 international society for heart and lung transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 32: 157-187. [Crossref]
- On The Origin of Species By Means of Natural Selection. Charles Darwin. 1859.

