Baroreflex failure (BF) is a rare disorder that causes fluctuations of BP with episodes of severe hypertension (HT) and elevated heart rate (HR) in response to stress, exercise, and pain. Symptoms may include headache, sweating, and high HR that does not respond to medications. Treatment is complex and usually involves medications to control BP and HR along with stress reduction techniques.
We report the case of a 24-year-old man referred to our center for volatile HT and HR. Six months before he experienced a severe trauma of cervical spine C5. He undergoes surgical reparation but retained a quadriplegia and both BP and HR became highly volatile. The clinical; laboratory and echocardiography evaluations ruled out any other secondary causes of HT and elevated HR. The patient underwent an exploration of the autonomic nervous system which revealed a beta central and peripheral sympathetic hyperactivity; associated with a patentee anomaly of the baroreceptors with important fluctuation of the arterial pressure above 20 mmHg at rest and high HR in the basal state (112 bpm). As therapeutic suggestion, our patient was put on phenobarbital and beta-blocker at low dose with a marked clinical improvement.
This case study demonstrates the effects of BF, which presumably occurred secondary to a cervical trauma. The interesting features are:
- The clinical syndrome of paroxysmal hypertension, headache, sweating and a high HR in BF.
- The difficulty of diagnosis of BF even with the aid of autonomic tests and the real therapeutic challenge open to broad future prospects.
baroreflex failure, hypertension, heart rate, cervical trauma, autonomic tests
The autonomic nervous system plays an important role in the minute-to-minute regulation of blood pressure (BP). This function depends largely on the baroreflex. The afferent limb of this reflex begins with pressoreceptors located in the carotid sinus and aortic arch. Its first synapse is located in the nucleus tractus solitarii (NTS) where, in connection with other brain stem centers, sympathetic and parasympathetic outflow is regulated. This is an extremely effective system that reacts to changes in BP in seconds and maintains it within a relatively narrow range.
In no instance is the effectiveness of the baroreflex more evident than in its absence [1].
Baroreflex failure (BF) is a rare disorder that causes fluctuations of BP with episodes of severe hypertension (HT) and elevated heart rate (HR) in response to stress, exercise, and pain.;
Individuals can also have hypotension with normal or reduced HR during periods of rest [2,3]. Symptoms of BF may include headache, sweating, and a high HR that does not respond to medications [3]. However, a complete history and physical is necessary because BF may resemble another rare disorder called Pheochromocytoma. The onset of BF can be very abrupt or more gradual.
In many cases, the causes are not known. However, BF can result from surgery or radiation treatment for cancers of the neck, injury to the nerves involved in sensing BP, or a degenerative neurologic disease [2,3].
Treatment usually involves medications to control BP and HR along with stress reduction techniques [3].With time, the pressor peaks may attenuate somewhat and the depressor valleys may become a greater problem for the patient, but these changes in the character of baroreflex failure are often played out over many years. Nevertheless, because of the complexity of treating these polar shifts in blood pressure, frequent follow-up of patients is important to make certain they are at an optimal regimen at all times [4].
In this study, we reported an example of BF due to a cervical trauma which is a very rare case. Such patient is a diagnostic and a therapeutic challenge.
A 24-year-old man was referred to our center for evaluation of possible baroreflex failure. Her chief symptom was volatile HT and HR. Six months before admission, the patient experienced a neck trauma during a football playing accident which resulted in severe trauma to the cervical spine C5 (Figure 1). He Undergoes surgical reparation of his cervical spinal (Osteosynthesis C4-C6 with implantation of iliac graft) (Figure 2).
After this accident, the patient retained a quadriplegia and both BP and HR became highly volatile. Systolic BP (SBP) values as high as 210 mmHg had been recorded. The hypertension was exacerbated by psychological stress and minor physical activity such that the formerly active man was restricted to his home because of his paralysis. He also noted symptomatic hypotension with BP readings of 60/30 mm Hg. we did not have any idea of orthostatic symptoms since he can’t stand up; however he did not report any other symptoms of autonomic dysfunction. The physical examination demonstrated that the patient was in good condition with a body weight 70 kg. There was no absent or weak limb pulses, heart murmurs or abdominal bruits. The clinical and laboratory evaluations for primary aldosteronism, pheochromocytoma or other secondary causes of arterial hypertension and hypotension had been carefully ruled out. The echocardiography and global longitudinal strain of the patient were normal (Figure 3).
In the iterative EKG (Electrogardiograms) performed, tachycardia was detected at110- 145 beats per minute (bpm) (Figure 4).
The patient underwent an exploration of the autonomic nervous system which revealed the following autonomic profile:
- A sympathetic hyperactivity beta central (at 16%, more than 10% in normal individuals)
- A sympathetic hyperactivity beta peripheral (at 31%, more than 10% in normal individuals)
- A normal sympathetic alpha central response (at 10%).
- A sympathetic peripheral alpha deficiency (at 7%, less than 10% in normal individuals)
- A well-preserved vagal response in deep breathing (at 30%).
- Associated with a patentee anomaly of the baroreceptors with important fluctuation of the arterial pressure above 20 mmHg (from 145/100mmHg to 168/118 mmHg) at rest and a very high HR in the basal state (112 bpm).
The hand grip and the orthostatic test could not be practiced considering his tetraplegia. The orthostatic test was replaced by inclinations in left lateral decubitus then right and which showed a dramatic variation in BP with a drop in the TA in DLG to 94/38 mmhg.
As therapeutic suggestion, our patient was put on phenobarbital and beta-blocker at low dose with a marked clinical improvement.

Figure 1. (a) Superior view of Heart A slab demonstrating nerve staining within the mitral valve, the surviving cusp of the aortic valve, and a coronary artery; (b) Magnified superior view of Heart A slab highlighting darker nerve stain in the mitral valves distal regions and within the cusp of the aortic valve (arrows); (c) Inferior view of the Heart A slab showing the anterior leaflet of the mitral valve (arrow) with clear nerve stain in the distal regions tapering off down towards the chordae tendineae; (d) Superior view of second Heart A slab showing dark nerve staining along the rim of the exposed coronary artery (arrow).
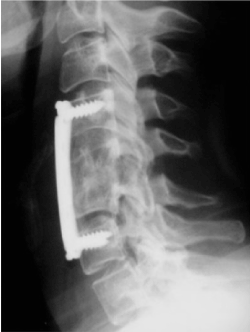
Figure 2. (a) Anterior view of Heart B; (b) Posterior view of Heart B right atrium; (c) Posterior view of Heart B left atrium; (d) Anterior view of Heart B right ventricle; (e) Posterior view of Heart B left ventricle; (f) Anterior view of Heart C; (g) Posterior view of Heart C right atrium; (h) Posterior view of Heart C left atrium; (i) Anterior view of Heart C right ventricle; (j) Posterior view of Heart C left ventricle.
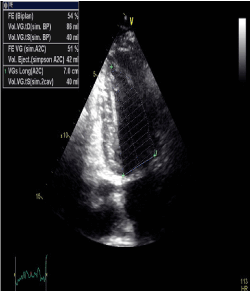
Figure 3A. (a) Anterior view of Heart B denoting light staining along the left anterior descending artery; (b) Anterior view of Heart C with a distinct patch of nerve staining on the proximal portion of the left anterior descending artery (arrow) in addition to light staining throughout the vessels; (c) Superior view of Heart B right atrium with sizeable nerve staining of the AV node center left (arrow); (d) Superior view of Heart C right atrium with small, discrete nerve staining of the AV node upper left (arrow); (e) Posterior view of Heart B greater vessels with nerve staining correlating to cardiac plexus ganglia (arrow); (f) Posterior view of Heart C greater vessels showing rough, black patches of unknown tissue in addition to nerve staining along the rim of the right pulmonary artery in the lower right (arrows).
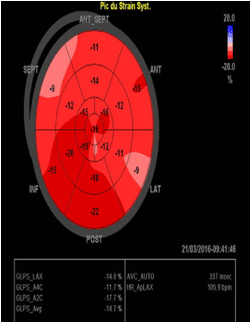
Figure 3B. (a) Anterior view of Heart B interior of the aorta; (b) Anterior view of Heart C interior of the aorta; (c) Superior view of Heart B mitral valve anterior leaflet; (d) Superior view of Heart C mitral valve anterior leaflet; (e) Anterior view of Heart B aortic valve cusps with inferior view of anterior leaflet of the mitral valve; (f) Anterior view of Heart C aortic valve cusps with inferior view of anterior leaflet of the mitral valve.
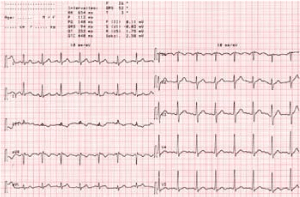
Figure 4. (a) Anterior view of Heart B denoting light staining along the left anterior descending artery; (b) Anterior view of Heart C with a distinct patch of nerve staining on the proximal portion of the left anterior descending artery (arrow) in addition to light staining throughout the vessels; (c) Superior view of Heart B right atrium with sizeable nerve staining of the AV node center left (arrow); (d) Superior view of Heart C right atrium with small, discrete nerve staining of the AV node upper left (arrow); (e) Posterior view of Heart B greater vessels with nerve staining correlating to cardiac plexus ganglia (arrow); (f) Posterior view of Heart C greater vessels showing rough, black patches of unknown tissue in addition to nerve staining along the rim of the right pulmonary artery in the lower right (arrows).
Baroreflexes have an important role in BP regulation. Changes in BP elicit changes in stretch of carotid and aortic baroreceptors. The altered baroreceptor stretch is conveyed to medullary brain stem nuclei via the glossopharyngeal and vagus nerves. The primary afferent relay stations in the brain stem are the nuclei tractus solitarii (NTS). In the brain stem, information from baroreceptors is integrated with input from other afferents and cortical input. Efferent parasympathetic and sympathetic activity are adjusted to compensate for the change in systemic BP. Thus, the baroreflex attenuates excessive BP changes. This mechanism serves to maintain blood flow to the organs, especially the brain [1] (Figure 5).
There is a large body of literature on baroreflex function in different animal species and in humans. However, the number of BF patients reported in the literature is relatively small. Impaired baroreflex function seems to be common after radiation therapy for laryngeal cancer and after unilateral carotid endarterectomy. However, overt BF appears to be uncommon in both conditions [5,6]. The small number of reported cases may suggest that BF is a rare condition. Perhaps the probability to experience bilateral damage to afferent baroreflex structures is low. An alternative explanation for the small number of reported cases is that many cases of baroreflex failure go undetected.
In most patients, the mechanism that led to bilateral interruption in afferent baroreflex input is suggested by the history [7]. A common cause of BF is extensive neck surgery and radiation therapy for cancers of the neck, which may damage baroreceptors or afferent baroreflex neurons [7-9]. In some patients, the bilateral loss results from repeated trauma to the neck [10]. BF has also been described in patients with the familial paraganglioma syndrome[11,12]. In another patient, BF was secondary to paraneoplastic encephalomyelitis [13]. However, in a number of patients with typical signs and symptoms of BF, no etiology could be documented.
Most patients who are ultimately diagnosed with BF are carried out at the exploration unit of the autonomic nervous system (ANS), of cardiology service “A” at Ibn Sina University Hospital. These patients were referred by hypertension specialist physicians to study their ANS in which their hypertensive episodes are usually accompanied by tachycardia, a so-called “tracking” of BP and heart rate [7,9].
Many patients experience sensations of warmth or flushing, palpitations, headache, and diaphoresis. The hypertensive episodes are triggered by factors such as psychological stress, physical exercise, and pain. A minority of patients present with episodes of hypotension and bradycardia. Hypotensive episodes can be observed when patients are resting and cortical input is diminished. Otherwise, severe Hypotension (OH) is not a typical symptom of BF.
OH may be observed in BF patients who are volume depleted or treated with sympatholytic drugs. The onset of BF can be very abrupt or more gradual. An abrupt onset of symptoms typically occurs in patients with BF attributable to neck surgery. Arriving at the correct diagnosis may be straightforward in this group.
BF with a more gradual onset as a consequence of radiation therapy or neuronal degeneration may be difficult to diagnose. The degree of HTA seems to be different during the acute and the chronic phase of the disease. After acute interruption of afferent baroreflex input, BP is particularly high [14,15]. Apneic spells can be seen during the first 24 hours, when carotid body input to the central nervous system (CNS) is lost. In the more chronic phase, the average BP tends to decrease. Yet BP remains highly variable. A similar time effect has because of the loss of baroreflex buffering, patients with impaired baroreflex function are extremely hypersensitive to vasoactive medications, thus, standard doses of anti-hypertensives may cause a profound depressor response.
The pressor agents, which may be contained in over-the-counter medications or dietary supplements, can raise BP to dangerously high levels. BF patients are extremely volume sensitive. Volume loss worsens hypotensive episodes. Volume expansion has the opposite effect.
Simple cardiovascular autonomic tests, such as determination of respiratory sinus arrhythmia, a Valsalva maneuver, and cold-pressor and handgrip testing, can be helpful to further elucidate the pathophysiology. Sympathetic efferents to the vasculature and to the heart are intact in BF patients. Therefore, these patients exhibit a normal or even an increased pressor response to cold-pressor and handgrip testing. In contrast, these responses are attenuated in patients with autonomic failure.
The first step in the treatment of patients with impaired baroreflex function is the education of the patient, family members, and the referring physicians. In addition, the patients and family members must be trained how to measure BP.
It is particularly important to convey the information that many medications that do not elicit changes in BP in healthy subjects may have a dramatic effect in BF patients. Therefore, vasodilating drugs such as calcium channel blockers should be avoided in BF patients [2]. Also, the medications that change sympathetic activity or vascular tone must be used with great caution in susceptible individuals.
Changes in sodium balance may shift the average BP to higher or lower values in patients with BF. Therefore, antihypertensive therapy with diuretics cannot be recommended for the majority of patients.
In particular, patients with selective BF experience hypotensive episodes [9]. Sometimes, the HT is acutely exacerbated by the antihypertensive. In selected patients, pharmacological treatment of the hypotension is required, and because of its long duration of action, fludrocortisones is a good choice for the treatment of hypotension in these patients.
Benzodiazepines also can be used in selected patients; they elicit a reduction in BP and are particularly useful in the acute phase of BF.
Also, Phenobarbital (PhB) has shown its effectiveness in the treatment of autonomic dysregulation disorders and can thus be used in this case. Long-term treatment reduces the sympathetic hyperactivity encountered during tests for stimulating the sympathetic system. The functional clinical improvement as well as the reduction of sympathetic hyperactivity, which was the goal of the sympathetic stimulation tests using low doses of phenobarbital was remarkable in the preliminary test. Thus, the invention makes it possible to provide a novel pharmacological prescription for treating autonomic dysregulations such as sympathetic hyperactivity [16].
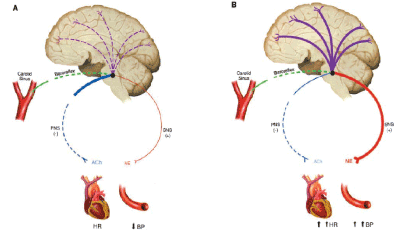
Figure 5. (a) Anterior view of Heart B interior of the aorta; (b) Anterior view of Heart C interior of the aorta; (c) Superior view of Heart B mitral valve anterior leaflet; (d) Superior view of Heart C mitral valve anterior leaflet; (e) Anterior view of Heart B aortic valve cusps with inferior view of anterior leaflet of the mitral valve; (f) Anterior view of Heart C aortic valve cusps with inferior view of anterior leaflet of the mitral valve.
This case study demonstrates the effects of BF, which presumably occurred secondary to a cervical trauma. The interesting features are:
- The demonstration of the absence of arterial baroreceptors reflex control of heart rate and efferent muscle sympathetic nerve activity in patients with BF.
- Importance of the cardiopulmonary baroreceptors reflex control of sympathetic nerve activity
- The lack of sustained hypertension, and the clinical syndrome of paroxysmal hypertension, headache, sweating and a high HR in BF.
- The difficulty of diagnosis even with the aid of autonomic tests.
- BF is a real therapeutic challenge open to broad future prospects.
- Biaggioni I, Whetsell WO, Jobe J and Nadeau JH (1994) Baroreflex failure in a patient with central nervous system lesions involving the nucleus tractus solitarii. Hypertension 23: 491-495. [Crossref]
- Heusser K (2005) Hypertension Grand Rounds: Baroreflex Failure. American Heart Association.
- Autonomic Disorders Consortium. Baroreflex Failure. Rare Diseases Clinical Research Network (RDCRN).
- Ketch T, Biaggioni I, Robertson R, Robertson D (2002) Four Faces of Baroreflex Failure Hypertensive Crisis, Volatile Hypertension, Orthostatic Tachycardia, and Malignant Vagotonia. Circulation 105: 2518-2523. [Crossref]
- Timmers HJ, Karemaker JM, Wieling W, Kaanders JH, Folgering HT, et al. (2002) Arterial baroreflex and peripheral chemoreflex function after radiotherapy for laryngeal or pharyngeal cancer. Int J Radiat Oncol Biol Phys 53: 1203-1210. [Crossref]
- Timmers HJ, Buskens FG, Wieling W, Karemaker JM, Lenders JW (2004) Long-term effects of unilateral carotid endarterectomy on arterial baroreflex function. Clin Auton Res 14: 72-79. [Crossref]
- Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda-Garcia R, et al. (1993) The diagnosis and treatment of baroreflex failure. N Engl J Med 329: 1449-1455. [Crossref]
- Sharabi Y, Dendi R, Holmes C, Goldstein DS (2003) Baroreflex failure as a late sequela of neck irradiation. Hypertension 42: 110-116. [Crossref]
- Timmers HJ, Karemaker JM, Lenders JW, Wieling W (1999) Baroreflex failure following radiation therapy for nasopharyngeal carcinoma. Clin AutonRes 9: 317-324. [Crossref]
- Jordan J, Shannon JR, Black BK, Costa F, Ertl AC, et al. (1997) Malignant vagotonia due to selective baroreflex failure. Hypertension 30: 1072-1077. [Crossref]
- Biaggioni I, Whetsell WO, Jobe J, Nadeau JH (1994) Baroreflex failure in a patient with central nervous system lesions involving the nucleus tractussolitarii. Hypertension 23: 491- 495. [Crossref]
- Phillips AM, Jardine DL, Parkin PJ, Hughes T, Ikram H (2000) Brain stem stroke causing baroreflex failure and paroxysmal hypertension. Stroke 31: 1997-2001. [Crossref]
- Jardine DL, Krediet CP, and Robinson BA (2004) Baroreflex failure secondary to paraneoplastic encephalomyelitis in a 17-year-old woman with neuroblastoma. J Neurol Neurosurg Psychiatry 75: 1650 -1651. [Crossref]
- Lampen H (1949) Experimenteller entzu¨gelungshochdruck bei arterieller hypertonie. Zeitschrift fu¨r Kreislaufforschung 38: 577-592.
- Fagius J, Wallin BG, Sundlöf G, Nerhed C, Englesson S (1985) Sympathetic outflow in man after anaesthesia of the glossopharyngeal and vagus nerves. Brain 108: 423-438. [Crossref]
- Benjelloun H (2013) Use of phenobarbital (PHB) in small doses in the treatment of autonomic dysregulations such as sympathetic hyperactivity. (WO2013154410).






