Abstract
Although Bacillus amyloliquefaciens has never been reported as human pathogen to our knowledge, we described two cases of neonatal bacteremia due to Bacillus amyloliquefaciens in preterm neonates, 22-day-old and 56-day-old, who were successfully treated.
Key words
Bacillus amyloliquefaciens, nosocomial infection, preterm neonate
Introduction
Over a two-week period in august 2012, Bacillus amyloliquefaciens was isolated from blood cultures from two preterm neonates who were hospitalized in our intensive care unit. They both developed an inflammatory syndrome and were treated for atypical necrotizing enterocolitis, Bell classification stage 1.
Case report
The first one, a 1170 g male infant, second twin, was born by caesarian section with cephalic presentation after 28 weeks and 5 days of gestation in July 2012. Prior to delivery, his mother received amoxicillin for prolonged premature rupture of membranes. Except for premature labor, her pregnancy had been otherwise normal. The infant had respiratory distress immediately after birth and was treated with mechanical ventilation and exogenous surfactant. A central catheter was inserted. He received empirical cefotaxim, amoxicillin and amikacin. Blood cultures obtained on admission to the neonatal intensive care unit gave negative results and antibiotics were discontinued after 48 hours. On postnatal day 23, the infant suffered from an inflammatory syndrome with an elevated C reactive protein (5.2 mg/dL) treated with empirical meticillin, gentamicin and central catheter withdrawal for suspected catheter related bloodstream infections. Peripheral and central blood culture and catheter culture were positive to Bacillus spp. Antibiotic treatment was switched to cefotaxim for 5 days. On postnatal day 28, blood culture was still positive to Bacillus spp but the infant wasn’t symptomatic anymore and CRP was under 0.3 mg/dL. No further treatments were given. We should notice that he was fed to breast and no artificial milk was used before the inflammatory syndrome. On postnatal day 40, the infant became hypotensive with massive regurgitation and CRP was growing up to 7.9 mg/dL and procalcitonin to 2.6µg/L. Initial symptoms included feeding intolerance, increased gastric residuals, abdominal distension but no bloody stools nor intestinal pneumatosis. An empirical antibiotic therapy was begun with metronidazole, cefotaxim, vancomycin and amikacin for suspected necrotizing enterocolitis and maintained for 7 days. The control blood culture was negative (CRP < 0.3 mg/dL). He was discharged home on day 81 of hospitalization.
The second one, a 1450 g female infant, was born by spontaneous vaginal delivery after a 30 week gestation in June 2012. Prior to delivery, her mother received amoxicillin for an inflammatory syndrome (CRP > 111 mg/L) and a leukocyte count of 23.12 G/L. Except for premature labor, her pregnancy had been otherwise normal. The infant had respiratory distress immediately after birth and was treated with mechanical ventilation, exogenous surfactant, steroids. She received empirical cefotaxim, amoxicillin, and amikacin for 2 days for an initial CRP up to 1.1 mg/dL and mother-child infection. After, antibiotics were switched to amoxicillin for 7 days for a positive Streptococcus B vaginal specimen. Placenta was positive to Streptococcus B too and tracheal tube was positive to gram negative bacteria. On postnatal day 37, the infant had respiratory distress, bloody stools and CRP was growing up to 10.2 mg/dL and procalcitonin to 1.08 µg/L. Initial symptoms included also feeding intolerance. An empirical antibiotic therapy was begun with amikacin for 48 hours and metronidazole and cefotaxim for 7 days for suspected necrotizing enterocolitis which encouraged us to stop the nutrition for 18 days. Initial blood culture was negative. On postnatal day 57, the infant had a sepsis on central catheter infection treated with amikacin, cefotaxim and vancomycin (CRP 9,9,3) for 7 days. Peripheral and central blood cultures were positive to Bacillus spp. The central catheter was withdrawn on day 63 and his culture was sterile. She was discharged home on day 68 of hospitalization.
These two preterm neonates were admitted in the same unit, in two neighbored rooms for 8 consecutive days (Figure 1). Medical and non-medical personal was taking care of both of these patients. The two strains of Bacillus spp were checked by AP-PCR (Arbitrarily Primed polymerase chain reaction) and were found to be cloned strains of Bacillus amyloliquefaciens which lead us to the primer conclusion of a hand transmission linked to central catheter infection.
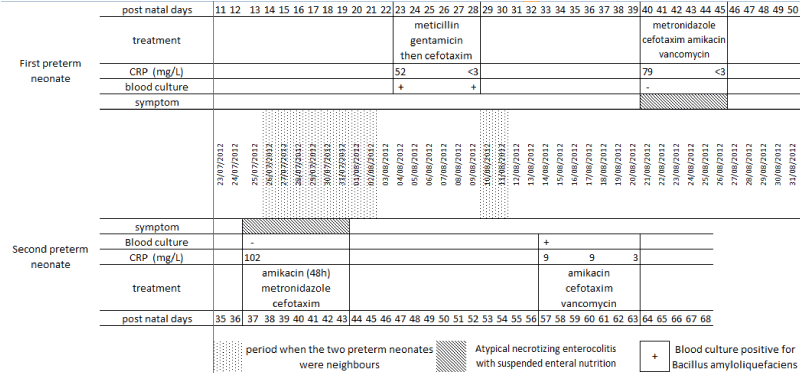
Figure 1: Characteristics of the two cases: treatment, biology, symptoms and spatio-temporal relationship between them.
Discussion
Bacillus species are widely distributed in nature and well known in the food industry as probiotics [1] and due to the ability of many strains to produce enterotoxins, a topic which has been reviewed recently [2-5]. Bacillus amyloliquefaciens, a gram-positive, rod-shaped, oval spore forming, aerobe bacterium of the family Bacillaceae, that is found commonly in soil, air [6], food, such as dried milk products [7], and many other sources, is the main strain implicated in production of α-amylase and protease [8,9]. Few studies also described this organism found on the hands of nursing staff or alcoholic preparation [10]. Also known for his use in biocontrol products [11,12] and probiotics [13], Bacillus amyloliquefaciens is not known as a human pathogen but, in recent years, there has been an increasing appreciation for the potential of Bacillus as opportunistic pathogens in immune-compromised [6,14] or otherwise critically ill patients, those with foreign bodies [15] and intravenous drug abusers as seen with B. cereus [16,17], B. thuringiensis [10] and B. anthracis [18]. Some gastrointestinal infections are also reported with Bacillus species [19,20].
Advances in neonatal intensive care over the past several years have improved survival of very low birth weight neonates, but due to their immature immune systems as well as their being subjected to prolonged mechanical ventilation and frequent use of long term intravascular catheter, members of this vulnerable population are particularly susceptible to disseminated disease caused by environmental organisms such as Bacillus [6,7,14, 15,18-21].
There are no documented cases of clinically significant invasive infection in neonates due to B. amyloliquefaciens as the significance of the isolation of B. amyloliquefaciens is not appreciate from clinical specimens obtained from newborns due to its lack of appearance as an already known nosocomial pathogen [1,22]. The significance is further clouded by the frequent appearance of the species in cultures as a contaminant [23].
Despite the small number of reported cases of Bacillus systemic infections in preterm neonates, it seems likely that the actual occurrence of this organism is more common as a true pathogen than the published literature would suggest. This belief is based on the fact that clinical laboratories may not attempt to determine the complete species identification of Bacillus organisms arbitrarily designating them as contaminants without adequate consultation with clinicians [21]. Another point would be, as suggested before, the confusing taxonomy of the genus Bacillus which may have been responsible for the inappropriate attribution of infections to B. cereus even if they were due to others species such as B. subtilis. To add with that, differences between some of the species of the genus Bacillus remained unclear for a long time and, as an example, B. amyloliquefaciens is a distinct entity only since 1980, before that, it wasn’t distinguished from other named species of Bacillus [8,24].
Assessment of the origin of infections due to organisms such as Bacillus that are widely disseminated in the environment is often difficult and may not yield an obvious source. To investigate the possibility of a common source and extent of the dissemination, an environmental research has been done in our units but remained negative. Food and floor sources were researched and furthermore, antiseptics and alcoholic preparation were also checked. Procedure related to room ventilation wasn’t monitored due to the small number of cases, the lack of respiratory manifestation and the lack of positive result on tracheal tube. A food contamination has not totally been excluded due to the use of B. amyloliquefaciens in food industry but symptoms may appear 10 to 14 h following ingestion of foodstuffs contaminated with enterotoxigenic strains. Foods most often implicated in the diarrheal syndrome include poultry, cooked meats, soups, desserts, and occasionally fluid and dry milk products [7].
The first preterm neonate has been successfully treated with meticillin, gentamicin and then cefotaxim for 5 days. The second has been successfully treated with vancomycin for 4 days and amikacin, cefotaxim for 7 days. An antibiogram was made and revealed that the pathogen had no resistance to any of those antibiotics despite an increased number of resistance to vancomycin described in the literature [25]. Bacillus species are also able to form biofilm which could help them to survive in a hospital environment, which lead us to be even more careful about these therapies [10]. To add with that, Bacillus species can easily adhere to the surface of catheter and this property makes central catheter withdrawal primordial to be as efficient as possible.
References
- Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J (2005) Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med 201: 1479-1486. [Crossref]
- Guimarães JAC (2011) Pathogenesis of systemic juvenile idiopathic arthritis: role of innate immunity [master’s thesis]. Porto (PT): Porto University.
- Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, et al. (2005) Therapeutic efficacy of humanized recombinant anti–interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 52: 818-825. [Crossref]
- Ferreira CS, Ribeir2021 Copyright OAT. All rights reserv, Freire M, et al. (2007) Síndrome de Evans na evolução da doença de Still. Rev Pediatr Moderna 43: 327-328.
- Carvalho RL, Tavares CM, Kuschnir MC, Aquino JHW (2007) Doença de Still na adolescência. Adolesc Saúde 4:53-55.
- Rosmaninho I, Guerra P, Guerra A, Brito I (2003) Artrite idiopática juvenil – forma sistêmica. Um caso particular. Acta Pediatr Port 34: 125-127.
- Ramos VCS, Ronchezel MV, Okuda EM, Sacchetti SB (2006) Caracterização epidemiológica, clínica e laboratorial de 100 crianças com artrite reumatóide juvenil. Rev Paul de Pediatr 24: 335-342.
- Bywaters EG (1971) Still's disease in the adult. Ann Rheum Dis 30: 121-133. [Crossref]
- Hadchouel M, Prieur AM, Griscelli C (1985) Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: possible relationship to drugs or infection. J Pediatr 106: 561-566. [Crossref]
- Sen V, Ece A, Uluca U, Günes A, Yel S, et al. (2015) Evaluation of children with juvenile idiopathic arthritis in southeastern Turkey: a single center experience. Hippokratia 19: 63-68. [Crossref]
- Pasqualotto FS, Ramos MD, Moussalem GF, Fortunato M, Assis SB, et al. (2009) Ativação macrofágica (SAM) em um paciente de 14 anos. Rev Biodiv 8:78-79.

