Abstract
Narrowed, uterine arterioles in the placental bed are the histologic hallmark of many, clinical obstetric syndromes including preeclampsia, IUGR, preterm labor, midtrimester loss, and, placental abruption. Early reports (1945-1980) illustrate, but do not explain, accompanying “halos” of hyalinised cells around these narrowed arterioles that are consistent features of these lesions (Figure 1a). Similar, narrowed arterioles are common in many gynecologic syndromes where there are “halos” of regenerating, injured nerves around narrowed uterine arterioles in presentations of chronic pelvic pain, dysmenorrhea, endometriosis , dyspareunia, vulvodynia, irritative bladder, and, bowel syndromes (Figure 1b). The neurovascular injury is one, and the same, in both obstetric and gynecologic, clinical syndromes. It results from injuries to pelvic vasomotor nerves (the “neural” injury) that release neural cytokines and growth factors causing transmural hyperplasia and narrowing of denervated arterioles, that become susceptible to successive, hyaline and fibrinoid changes (the “vascular” injury). Their common origins have been difficult to recognise because during pregnancy the injured arterioles extend to the placental bed, but, the injured nerves do not, leaving one, or more, layers of hyalinised cells around the injured arteriole in the placental bed biopsy (Figures 1a-b). Injuries to pelvic nerves result from “difficult” first labors, physical efforts during defecation, complications of gynecologic surgical procedures, as well as hypertension. Injuries to arteriolar vasomotor nerves induce “new” and “novel” receptors including purinergic, P2X3 and VEGF receptors in arteriolar smooth muscle that may respond to “pain” and “stretch” , or, activate other autonomic reflexes. Recent evidence suggests that the unexpectedly diverse, and varying, consequences of these neurovascular injuries may contribute to the pathophysiology of different, obstetric and gynaecologic syndromes.
Key words
premenstrual, pelvic pain, autonomic nerves, P2X3 receptors, VEGF receptors, hyperalgesia, allodynia
Introduction
In 1937, Drs Alan Moritz and Mary Oldt described narrowing of arterioles associated with hyperplasia of the tunica media and intima in kidney, spleen, pancreas and adrenals in a series of 100 hypertensive, post-mortem subjects (Figure 1) [1]. In 1945, Dr AT Hertig (Harvard, MA, 1945) described narrowed uterine arterioles in preeclampsia and placental abruption (Figure 2a) [2]. Since those initial reports, many groups have reported similar narrowed, uterine arterioles in preeclampsia [3-7]. However, in many of the accompanying illustrations there are perivascular “halos” of hyalinised cells (Figure 2a) surrounding the narrowed arterioles that did not attract comment, or explanation, in the initial reports (1945 onwards). More recently, Professor Brosens and Romero have broadened the description of these narrowed arterioles to include many other important, clinical obstetric syndromes including intrauterine growth retardation, placental abruption, preterm labor, preterm PROM and midtrimester loss [8-10]. Narrowed uterine arterioles in the placental bed are not a specific, histologic finding.
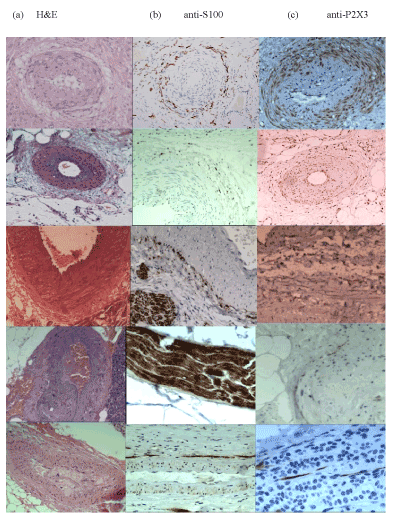
Figure 1. Visceral arteriolar injuries in pregnant, and, non-pregnant, hypertension
Widespread visceral arteriolar stenosis in hypertension was originally described by Dr A Moritz & M Oldt, 1937 (Cleveland, OH.) in kidney, spleen, pancreas and adrenals (2-5 A-C), though similar injuries may take place in the uterus in preeclampsia (1A-C) with, or without, HELLP syndrome .
Row 1 uterus, Row 2 kidney, Row 3 spleen, Row 4 pancreas, row 5 adrenals,
Column (A) is stained with hematoxylin and eosin, Column (B) is stained with anti-S100 antibody, Column (C) is stained with anti-P2X3 antibody.
Row 1a shows narrowed uterine arteriole with a “halo of hyalinisation” in the obstetric syndromes (x100, HE)
Row 1b shows narrowed uterine arteriole with a “halo of injured nerves” in the gynaecologic syndromes (x100, anti-S100)
Row 1c shows narrowed uterine arteriole expressing P2X3, purinergic receptors in hyperplastic tunica media and intima (x100, anti-P2X3)
Row 2 a-c shows narrowed arterioles from the hilum of the kidney demonstrating injured, perivascular renal nerves and early expression of P2X3, purinergic receptors in hypertension.
Row 3a-c shows narrowed arterioles from the hilum of the spleen demonstrating multiple layers of injured perivascular nerves (x100 Figure 1.iii.b)
Row 4a-c shows narrowed arterioles from the pancreas including a pancreatic nerve bundle with a U-shaped “cutting artefact” in its superior border as well as authentic loss of nerve fibers throughout the nerve bundle.
Row 5a-c shows narrowed arterioles from adrenals demonstrating adrenal cortical hyperplasia at x100 and x200 associated with injured pericortical nerves in this specific form of hypertension. Compare with denervatory endometrial and myometrial hyperplasia in adenomyosis and leiomyoma respectively.
Working independently on gynecologic tissue samples, our group described a perivascular “halo of injured nerves” in women with different forms of chronic pelvic pain with, or without, “endometriosis”, dysmenorrhea, vulval pain, irritative bladder and bowel syndromes (Figure 2b) [11,12]. Comparing the immunohistochemical injuries suggests that the origins of the neural and vascular (neurovascular) injuries are one and the same; however the regenerating “halo” of injured nerves is not visible in the placental bed biopsies of the obstetric syndromes because the nerves do not extend to the placental bed; they remain in the isthmus of the uterus throughout pregnancy (Figures 2a and 2b) [13]. Understanding the causes and consequences of the combined neurovascular injury offers potential explanations for different, clinical, obstetric and gynecologic syndromes.
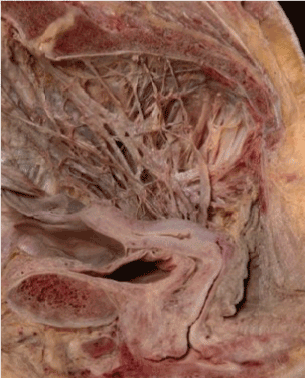
Figure 2. The pelvic autonomic nerves prosected and preserved in alcohol (not formalin) as described by Robert Lee, 1841 and Franz Frankenhauser, 1867
The pelvic autonomic nerves converge on the vaginal vault where they are vulnerable to injury during prolonged and persistent physical efforts during defecation, difficult first labours, and, some forms of gynaecologic surgery e.g. evacuation of the uterus. Pelvic sympathetic nerves (T10-L2) pass through the superior hyogastric plexus (1), hypogastric nerve (2), inferior hypogastric plexus (3), to enter the uterovaginal plexus of Frankenhauser (4), in combination with pelvic parasympathetic nerves from sacral segments S2-4 (5).
Early clinical accounts of uterine neurovascular injury
Dr HC Taylor (editor AJOG, 1953-1969) provided key insights into the etiology of premenstrual gynecologic symptoms in two series of patients that he studied in the 1940-50’s [14-18]. He thought the origins of the symptoms were “a disorder of the pelvic autonomic nervous system with a vascular component, occurring in psychiatrically predisposed individuals” [15]. He, further, suggested there was a “tendency to hyperalgesia in different areas of the pelvis supplied by the sensory components of visceral nerves”. Dr Taylor offered the deterioration of symptoms in the week before the onset of menstrual flow, their provocation by sexual intercourse, and, the finding of distended veins and amber fluid in the pouch of Douglas, as “clear evidence of a vascular disorder” [18]. Dr WM Allen (St Louis, MO), recorded similar findings at laparotomy in a smaller group of 28 patients with chronic pelvic pain, though he emphasised the accompanying, traumatic laceration of myofascial supports, notably the uterosacral ligaments (Figure 2) [19-20]. In his 1955 paper [19], Dr. Allen recorded chronic pelvic pain following a prior, significant, obstetric event in 22/28 cases, and occasionally, following cases of abortion and constipation (vide infra). At laparotomy, he made similar observations to Dr Taylor, describing amber fluid in the pouch of Douglas, unilateral or bilateral avulsions to the uterosacral and broad ligaments, along with uterine retroversion. Initially, he attempted to repair the myofascial injuries but reverted to hysterectomy after disappointing results [20]. In that second report, Dr Allen suggested that these injuries were sustained, for the most part, during difficult, intrapartum events. He had identified both the source and anatomic site of a putative, neural injury long before the widespread availability of laparoscopy and advanced, cross-sectional imaging techniques.
Obstetric and gynecologic “syndromes”
Professors Brosens and Romero have subsequently drawn attention to the association of the “great” obstetric syndromes with defective deep placentation, namely, the presence of “obstructive lesions” in the myometrial segments of the spiral arteries [5-10]. These collated reports describe similar, non-specific, vascular changes in preterm labor, preterm premature rupture of the membranes, placental abruption and midtrimester abortion [8-10]. Much attention has been given, historically, to events taking place during pregnancy including “mechanisms of endovascular trophoblast invasion” though less attention given to potential injuries taking place before pregnancy.
Our group has drawn attention to the similarity of the histologic appearances in gynecologic syndromes in chronic pelvic pain, “endometriosis”, vulval and vaginal pain, irritative bladder and bowel syndromes [21-30]. In all these syndromes the histologic appearances include perivascular nerve fiber proliferation (PVNFP) where there are one, or more, layers of injured, regenerating nerves around narrowed arterioles (Figure 1b-1d) [21]. Importantly, these may arise as a consequence of physical efforts during defecation, or, prolonged maternal voluntary efforts in the second stage of labor (among other obstetric injuries); both of which may lead to different, gynecologic syndromes [21,29]. Other groups have described denervation-reinnervation in different pelvic organs and syndromes [31-39], including endometriosis [40-50], though without necessarily describing the source of these injuries.
The pelvic autonomic nerves
Robert Lee described the morphology of the pelvic autonomic nerves in 1840, amidst considerable controversy [51-53]. Few of his colleagues, believed that they were looking at nerves in his prosections in alcohol (Figure 3). Not every contemporary colleague will be familiar with the detailed morphology of the pelvic autonomic nerves as they converge on the vaginal vault where they are vulnerable during difficult labors, gynecologic surgery requiring incision of, or, traction to the cervix, and, physical efforts during defecation (Figure 3) [54]. Post-1945 generations of medical students have been taught on cadavers embalmed in formalin that destroys, rather than demonstrates, autonomic nerves. The sympathetic (T10-L2) and parasympathetic nerve fibers (S2-4) pass through the uterosacral and broad ligaments in two key nerve bundles; the first runs through the uterosacral ligaments to supply the nerve plexus at the endometrial-myometrial nerve plexus; the second bundle enters the uterus with the uterine artery to supply the subserosal plexus. Both converge on a tubal plexus that supplies the proximal two thirds of the Fallopian tube whilst the distal third receives nerves from the ovarian plexus through the free edge of the mesosalpinx [55]. Ampullary ectopic pregnancy often arises from normal oocyte “pick-up” by the fimbriae but failure to transport the embryo along the Fallopian tube owing to an ipsilateral injury to the nerve supply of the proximal two thirds that is usually visible in the ipsilateral uterosacral ligament [28]. Recognising injuries to autonomic nerves as the source of clinical obstetric and gynecologic syndromes may have been impaired by loss of their morphology in formalin-embalmed cadavers however there has also been a problem with their microscopy. In Figure 1. Row 4b, there is a pancreatic nerve bundle demonstrating a “cutting artefact” on its superior border however there is clearly accompanying loss of at least four longitudinal nerve fibers in the nerve bundle. Many pathologists have collectively labelled these appearances as coincidental “cutting artefacts” when the loss of longitudinal nerve fibers is, in fact, a key indicator of distal denervation in that tissue section.
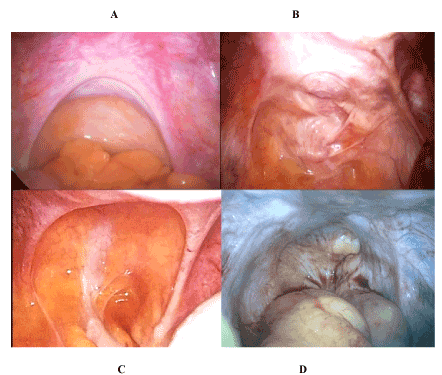
Figure 3. Different patterns of injuries to the uterosacral ligaments in different clinical circumstances, similar to myofascial injuries described originally by Dr WM Allen & Dr W Masters, 1955 (St Louis, MO)
A = normal, nulliparous, uterosacral ligaments contain uterine and tubal nerves that supply the endometrial-myometrial nerve plexus and the proximal two thirds of the Fallopian tubes
B = asymmetric injuries to uterosacral ligaments with, on the left, complete avulsion of uterine and sacral origins, and, on the right, scarring of the uterine insertion and probable, avulsion of sacral origin. These are typical of asymmetric forces during vaginal delivery.
C = symmetric injuries to uterosacral ligaments with bilateral avulsions of sacral origins. These, unusual appearances are typical of excessive uterine activity in early pregnancy e.g. following administration of misoprostol for miscarriage or unwanted pregnancy. Attenuation of the ligaments, without clear avulsion typically occurs following excessive uterine activity after administration of prostaglandins or intravenous oxytocin.
D = complete absence of uterosacral ligaments. These appearances are unusual in Western gynaecology though is not uncommon in China during the “one-child” policy. These injuries usually follow one, or more, second trimester abortions; they result in infertility, ectopic pregnancy or diffuse, symmetric adenomyosis in later life.
Causes of neural injury in the female pelvis
Childbirth
“Difficult”, nulliparous labors result in chaotic, neural injuries with both perivascular nerve fiber proliferation, and, some degree of stromal reinnervation (Table 1). Regenerating nerves re-grow over 4-5 years and become dense enough to result in hyperalgesia (or, allodynia) where “light touch causes pain or discomfort” [56]. Aberrant reinnervation has been described in every pelvic organ and some patterns of vulvodynia, dyspareunia, dysmenorrhea, frequency and urgency in response to bladder filling, or, irritative bowel symptoms, correspond to this formulation. In 2420 nulliparous women in the Avon Longitudinal Study of Pregnancy and Childbirth (ALSPAC, 1992) dataset there was doubling of self-assessed, “severe” gynecologic symptoms including chronic pelvic pain at 47 months post-delivery in women who had undergone “difficult” first labors (Table 1) [57]. That figure may significantly understate the size and extent of subsequent gynecologic problems since most women with chronic pelvic pain do not present until 5-8 years after their first baby. The laparoscopic phenotypes of parous women with chronic pelvic pain often demonstrate asymmetric, “traumatic laceration” of their myofascial supports, with, or without, deposits of ectopic endometrium (Figure 3). If the mother breastfeeds for six months then there will be no endometrium to adhere to asymmetric, pelvic myofascial injuries whereas if she bottle feeds then there may be ectopic endometrium available to define the injury [58]. There are often, similar, extensive neural injuries in the uterine isthmus and adjacent anatomic structures, irrespective of deposits of ectopic endometrium, though even these deposits of endometrium will develop reinnervation in mature lesions, justifying a surgical approach to some of these patients problems [41-43].
Table 1. Causes of injuries to pelvic autonomic nerves
- Childbirth
Difficulties in first labors are the most common cause of injuries to pelvic autonomic particularly as they pass through the uterosacral ligaments to insert into the posterior surfaces of the uterus and cervix. Induction of labor, prolonged labors (>12 hours), prolonged pushing (> 2 hours), big babies (>4000g), forceps deliveries, malpresentations, may all contribute to injuries to pelvic autonomic nerves |
- Constipation
In this context physical efforts during defecation, that affect 20-30% of Western urban populations, are a key source of pelvic neural injury (KW Heaton, 1993).It creates a characteristic, longitudinal injury in the neurovascular bundle where “new” nerves sprouting from the nerve bundle, adhere to the surface of the arteriole creating“perivascular nerve fiber proliferation” (GS Atwal, 2005).Women with these injuries present with “premenstrual” symptoms affecting any organ in the pelvis |
- “Contractions”
Excessive uterine activity complicating administration of utero tonic agents (10-40%) including oxytocin, misoprostol and prostaglandins, has devastating consequences on uterotubal nerves at different sites.They are injured; (a) in the body of the uterus and may contribute to some patterns of painful adenomyosis, (b) in the uterosacral ligaments that are the source of chronic pelvic pain in thin, attenuated ligaments. Excessive traction to the cervix may also avulse the nerve supply. |
Constipation
The relationship between some aspect of “constipation” - carefully defined, in this account, as “persistent, physical efforts during defecation” – and subsequent Western diseases, has a long, and controversial history.Sir William Arbuthnot Lane, Bt, wrote an important account of “chronic intestinal stasis” and its treatment by colectomy in 1924 however there have been subsequent, important physiologic contributions by DP Burkitt, FRS, epidemiologic studies by KW Heaton, FRCP, and, demonstration of immunohistochemical consequences by our group [21,59-62]. One percent of urban, Western populations only achieve defecation once per week, and, 0.1% only achieve defecation once per month [62]. Worse still, 20-30% percent require physical efforts to start or finish defecation; it is these physical efforts that cause “longitudinal” injuries to uterosacral ligaments (Figures 3a-3d), and, autonomic nerve bundles that result in regeneration of new injured nerves (“autonomic neurapraxis”) [21]. In nulliparous women with organ-specific, premenstrual symptoms e.g. dysmenorrhea, vulval pain, vaginal pain, irritative bowel and bladder syndromes there is a common histologic lesion that is caused for the most part, by a single neurologic injury (Figure 1b) [21,29]. These “new” nerves create concentric layers of injured nerves around pelvic arterioles maintaining close relationships with the adjacent, denervated and narrowed arteriole [21,23,29]. Increases in pelvic blood flow typically cause premenstrual pain that we label as different, clinical syndromes depending on the organ that has been injured (Figure 1b) [31-39].
Gynecologic surgery
The third cause of injuries to pelvic autonomic nerves is gynecologic surgery, most frequently, medical or surgical evacuation of the uterus [63,64]. Excessive traction to the cervix during surgical evacuation results in injuries to the uterosacral ligaments whereas hyperstimulation associated with administration of prostaglandins, or oxytocic agents, causes a similar injury to uterine nerves [64]. Over-vigorous curettage may also injure the nerve plexus at the endometrial-myometrial nerve plexus [30]. Clearly any surgical procedure that divides pelvic nerves may be the source of subsequent neurologic symptoms including LLETZ, cone biopsy, hysterectomy, colporrhaphy, etc. The key to understanding their pathophysiology is recognising the diverse and varying consequences of these autonomic injuries (Table 2).
Table 2. Consequences of injuries to pelvic autonomic nerves
- Hyperplasia
Denervation at different sites in the uterus results in different patterns of diffuse or localised hyperplasia that we term myoma or adenomyosis.Localised injury to the myometrium produces leiomyoma or adenomyoma; avulsion of the nerve supply results in diffuse, symmetric, painless, adenomyosis, injuries in the body of the uterus result in focal, asymmetric, painful adenomyosis. |
- Hypoplasia
Denervation-reinnervation of non-oestrogen dependent organs e.g. bladder and rectum, results in atrophy and pain e.g. interstitial cystitis, or, poor expulsion function of a sensitive rectum in rectal “hypersensitivity” |
- Impaired visceral function
Denervated uterus, bladder and rectum have reduced capacity to empty. Denervated cervix has an impaired capacity to dilate in labor, or, at hysteroscopy. |
- Impaired contractility
Denervated Fallopian tubes have impaired contractility that may contribute to infertility or ectopic pregnancy.Typically injuries to the nerve supply of the proximal 2/3 of the Fallopian tube (with intact ovarian innervation) allows fertilisation but results in ampullary ectopic pregnancy. |
- Opportunist infection
Denervation of the vulva and vagina results in opportunist infections e.g E.coli, bacterial vaginitis, vulvovaginal Candidiasis that may contribute to some forms of preterm labor, preterm PROM, and recurrent gynecologic infections |
- Pain
Depending on the stroma of the tissue, denervation results in different patterns of reinnervation, that results in “pain in response to light touch” (allodynia or hyperalgesia).The obvious example is vulval pain but the close relationship between injured pelvic autonomic nerves and arterioles results in widespread, premenstrual symptoms in many pelvic organs and their supports. |
- CNS “sensitisation”
Sustained neuropathic pain results in “storage” of that pain in the central nervous system.Post hysterectomy pain may reflect the underlying injury to the uterus that was. The reason for the procedure.Some sensory pelvic symptoms may reflect underlying neuromas in the “cut” uterosacral ligaments or vaginal cuff. |
Hypertension
Sustained high, intravascular pressures cause characteristic, pre-pregnancy injuries to uterine arterioles (Figure 2) [65,66]. Typically, there is aberrant reinnervation of injured vasomotor nerves though the histologic appearances differ slightly from the smooth “halo of hyalinisation” in Figure 2a. Instead of the smooth layer of hyalinisation there is a more disorganised appearance that looks like “woven baskets” surrounding the narrowed arterioles (Figure 2c). Similar appearances are apparent in other viscera including the kidney (Figure 1 Row 2b). These appearances are identical to those arising in the uterus in preeclampsia and other obstetric syndromes. Furthermore, it is now clear that women suffering from sustained preeclampsia, that causes fixed injuries to renal vasomotor nerves, may develop hypertension within a few years of their deliveries along with the potential for severe cardiovascular morbidity owing to the fixed injury to their renal circulation [67]. Attitudes to the timing of delivery in the hypertensive disorders of pregnancy may need to take into account the risks of remote consequences for the mother?
Consequences of neural injury in the female pelvis
Injuries to autonomic nerves carry a surprisingly wide range of pathophysiologic consequences (Table 2). In the uterus there is clear evidence of different patterns of endometrial hyperplasia (adenomyosis) [30] and myometrial hyperplasia (leiomyoma) [68-70] associated with different patterns of neural injury to the endometrial-myometrial nerve plexus and myometrium respectively, whereas in non-estrogen-sensitive tissues e.g. bladder and bowel, similar injuries result in atrophy. Uterotubal dysmotility results in retrograde menstruation, dysfunctional labor, subfertility and ectopic pregnancy [12,27,28]. Denervation of mucous membranes results in opportunist infection leading to a range of gynaecologic infection as well as contributing to some forms of preterm labor and PPROM [71]. Uterine arteriolar stenosis impairs fetal growth and injuries to vasomotor nerves cause viscero-visceral reflexes (uterorenal, hepatorenal and lienorenal) that cause cardiovascular adjustments that may result in preeclampsia, HELLP syndrome, and, subsequent, early-onset, postpartum hypertension [71]. Injured uterine nerves express TRPV-1 “pain” receptors, and, induce purinergic (P2X3) “stretch” and VEGF receptors in myometrium and arteriolar smooth muscle that may contribute to different patterns of vulvodynia, vaginal pain, chronic pelvic pain, irritative bladder and bowel syndromes, etc as well as central sensitisation leading to recurrent pain following surgery e.g. hysterectomy [72]. Viscero-visceral reflexes may also account for extrapelvic autonomic symptoms wherever there has been activation of pelvic autonomic injuries [73]. Many non-pregnant women with premenstrual syndromes may have dramatic extrapelvic symptoms in the week before menstruation owing to activation of injured nerves by increases in pelvic blood flow, whereas the weight of the pregnant uterus at 16-20 weeks gestation may contribute to pelvic pain with accompanying extrapelvic symptoms in multiparous women 4-5 years after a “difficult” first labor (Table 1).
Neurovascular origins of some gynecologic syndromes
Leiomyoma, adenomyosis and endometriosis all carry neurologic injuries. Savitskii described loss of nerves in some patterns of acquired leiomyoma in three papers in the 1980’s [68-70]. Diffuse, symmetric adenomyosis arises from loss of nerves at the endometrial-myometrial interface associated with bilateral injuries to the uterosacral ligaments (Figures 2a-2d) [74]. Less dramatic, neural injuries may cause different patterns of adenomyosis at different uterine sites as originally described by Thomas Cullen in 1908 [75]. Different patterns of “endometriosis” are associated with injuries to uterovaginal nerves that cause retrograde menstruation with adhesion of ectopic endometrium to sites of tissue injury e.g. symmetric, hypertrophied, uterosacral ligaments following years of physical efforts during defecation, or, asymmetric, attenuated, uterosacral ligaments following difficult intrapartum episodes [40-49]. The laparoscopic appearances are both, dichotomous and confusing – but if the surgeon ignores the ectopic endometrium, notes the myofascial injuries, and, asks the question “What injuries has this woman sustained, and, when did they occur ?” then (s)he may be able to provide a better explanation of their origins with improved advice to the patient [76] Both uteri demonstrate aberrant reinnervation throughout the anatomic structures at the junction of uterus and vagina that may include the isthmus of the uterus, cervix, upper vagina, uterosacral-cardinal ligaments, peritoneal surfaces and, even, the ectopic endometrium, itself [29,40-49]. The prevailing opinion remains that deposits of ectopic endometrium are the primary source of pelvic pain; though some believe that increases in pelvic blood flow on regenerating nerves expressing “new and novel” pain receptors may be a better explanation of symptoms. Vulvodynia, dyspareunia, dysmenorrhea, irritative bladder and bowel syndromes are all definable as “light touch causing pain or discomfort”, and, associated with different patterns of injury to pelvic nerves with similar pathophysiologic consequences and outcomes [30-39].
Neurovascular origins of some obstetric syndromes
Recognising that the neurovascular injury in the placental bed in the obstetric syndromes also results from injuries to uterine nerves is an important insight that may redirect etiologic attention in some of the common obstetric syndromes (Figures 1a-1b) [8-10,71]. Denervatory injuries to smooth muscle of arterioles and myometrium induces primitive, purinergic, P2X3 receptors that use ATP as a neurotransmitter (Figures 1e-1f) [77]. Stretching injured tissues including denervated arterioles releases ATP that stimulates reflex activity through uterorenal nerves that, in turn, causes a renal cortico-medullary vascular shunt [71]. Shutting down blood flow to the renal cortex activates the renin-angiotensin system and results in “early-onset” preeclampsia – that may be prevented, in part, by early administration (pre-16 weeks) of aspirin (Figures 4a-4d) [71,78]. Stretching injured myometrium may result in “late-onset” preeclampsia that is not prevented by aspirin [71]. Loss of uterorenal nerves as a result of sustained physical efforts during defecation may result in intrauterine growth retardation without activation of the uterorenal nerves, hypertension and proteinuria [71]. Denervation at any site in the lower genital tract will create the conditions for opportunist organisms to contribute to PPROM and preterm labor, and, rupture of the fibrinoid or hyaline “degenerations” of these vessels may result in placental abruption [71]. All are possible consequences of the original neurovascular injury [11,12]. As previously discussed, similar neurovascular injuries take place in kidney, spleen, adrenal and pancreas in hypertensive individuals [1,65,66], and, may contribute to the development of HELLP syndrome during pregnancy and early-onset, postpartum hypertension that leads to significant cardiovascular events [67].
Conclusions
Many clinical, obstetric and gynecologic syndromes develop from the remote consequences of autonomic neurovascular injuries. Injuries to pelvic vasomotor nerves result from “difficult” first labors, physical efforts during defecation, complications of common gynecologic, surgical procedures and hypertension. Clinical presentations depend on the site, nature and extent of the injury. In the UK, uterotubal neurovascular injuries frequently occur during “difficult” first labors and straining during defecation leading to “partial” injuries to nerve bundles that permit a pathway for regenerating injured nerves and subsequent painful, clinical syndromes of chronic pelvic pain, painful periods, painful sex, vulval pain, irritative bladder and bowel syndromes. In China, injuries to these pathways during midtrimester abortions tend to cause “complete” avulsion of the nerve bundles in the utrosacral ligaments leading to painless clinical presentations of infertility, ectopic pregnancy, and, advanced “painless” adenomyosis.
Preventing these neural injuries is clearly important since there appear to be few barriers to regenerating injured nerves that may cause pain and suffering at different times of life. Encouraging breastfeeding and appropriate toilet-training to establish a reliable infant diet and bowel habit are significant, preventive tasks. In the reproductive years, neuroprotective, intrapartum care and carefully-considered approaches to common gynecologic surgical procedures, are clearly important preventive approaches. Many post-reproductive careers continue to be marred by the direct, and indirect, consequences of childbirth decades after the original injuries. Preventing autonomic neurovascular injury becomes an important aspect of maintaining womens reproductive health and well-being over the course of their lifespans.
References
- Moritz AR, Oldt MR (1937) Arteriolar Sclerosis in Hypertensive and Non-Hypertensive Individuals.Am J Pathol13: 679-728. [Crossref]
- Hertig AT, In Toxemias of Pregnancy, Edited Kellogg FS. Clinics 1945; 4: 602-13.
- ZEEK PM, ASSALI NS (1950) Vascular changes in the decidua associated with eclamptogenic toxemia of pregnancy.Am J Clin Pathol20: 1099-1109. [Crossref]
- DIXON HG, ROBERTSON WB (1958) A study of the vessels of the placental bed in normotensive and hypertensive women.J Obstet Gynaecol Br Emp65: 803-809. [Crossref]
- Brosens I, Robertson WB, Dixon HG (1967) The physiological response of the vessels of the placental bed to normal pregnancy.J Pathol Bacteriol93: 569-579. [Crossref]
- Brosens IA, Robertson WB, Dixon HG (1970) The role of the spiral arteries in the pathogenesis of pre-eclampsia.J Pathol101: Pvi. [Crossref]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I (1980) Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy.Placenta1: 3-19. [Crossref]
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, et al. (1994) The preterm labor syndrome.Ann N Y Acad Sci734: 414-429. [Crossref]
- Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS (2011) Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae.Best Pract Res Clin Obstet Gynaecol25: 313-327. [Crossref]
- Brosens I, Pijnenborg R, Vercruysse L, Romero R (2011) The "Great Obstetrical Syndromes" are associated with disorders of deep placentation.Am J Obstet Gynecol204: 193-201. [Crossref]
- Quinn MJ (2016) The aetiology of narrowed uterine arterioles in obstetric and gynaecological syndromes.Placenta44: 114. [Crossref]
- Quinn MJ1 (2016) Autonomic denervation: A new aetiological framework for clinical obstetrics and gynaecology.Med Hypotheses89: 43-47. [Crossref]
- Wikland M, Lindblom B, Dahlström A, Haglid KG (1984) Structural and functional evidence for the denervation of human myometrium during pregnancy.Obstet Gynecol64: 503-509. [Crossref]
- Taylor HC Jr (1949) Vascular congestion and hyperemia; their effect on structure and function in the female reproductive system. Am J Obstet Gynecol 57: 211-230.
- Taylor HC Jr (1949) Vascular congestion and hyperemia; their effect on function and structure in the female reproductive organs; the clinical aspects of the congestion-fibrosis syndrome.Am J Obstet Gynecol57: 637-653.
- Taylor HC Jr (1949) Vascular congestion and hyperemia; their effect on function and structure in the female reproductive organs; the clinical aspects of the congestion-fibrosis syndrome.Am J Obstet Gynecol57: 637-653. [Crossref]
- Taylor HC Jr (1949) Vascular congestion and hyperemia; their effect on function and structure in the female reproductive organs; etiology and therapy.Am J Obstet Gynecol57: 654-668. [Crossref]
- Taylor HC (1951) [Pelvic disorders of autonomic origin in the female].Arch Gynakol180: 181-231. [Crossref]
- Allen WM, Masters WH (1955) Traumatic laceration of uterine support; the clinical syndrome and the operative treatment.Am J Obstet Gynecol70: 500-513. [Crossref]
- Allen WM (1971) Chronic pelvic congestion and pelvic pain.Am J Obstet Gynecol109: 198-202. [Crossref]
- Quinn MJ (2007) Perivascular nerve fibre proliferation: the consequence of prolonged straining during defaecation. J Obstet Gynaecol 27: 185.
- Quinn MJ, Slade RJ, Armstrong G (2006) Vaginal vault reinnervation and symptoms of genital prolapse.J Obstet Gynaecol26: 177-178. [Crossref]
- Quinn MJ, Kirk N (2002) Differences in uterine innervation at hysterectomy.Am J Obstet Gynecol187: 1515-1519. [Crossref]
- Quinn M, Kirk N (2004) Uterosacral nerve fibre proliferation in parous endometriosis. J Obstet Gynaecol 24: 189-190.
- Quinn MJ (2002) Interstitial cystitis--a time for revision of name and diagnostic criteria in the new millennium? BJU Int 89: 795.
- Quinn MJ (2008) Endometrial nerve fiber proliferation in "endometriosis".Am J Obstet Gynecol199: e13. [Crossref]
- Quinn MJ (2011) Endometriosis: the consequence of uterine denervation-reinnervation.Arch Gynecol Obstet284: 1423-1429. [Crossref]
- Zhang XM, Huang X, Xu H, Quinn MJ (2014) Altered innervation of the fallopian tube in ectopic pregnancy.J Obstet Gynaecol34: 531-532. [Crossref]
- Atwal G, du Plessis D, Armstrong G, Slade R, Quinn M (2005) Uterine innervation after hysterectomy for chronic pelvic pain with, and without, endometriosis.Am J Obstet Gynecol193: 1650-1655. [Crossref]
2021 Copyright OAT. All rights reserv
- Quinn M (2007) Uterine innervation in adenomyosis.J Obstet Gynaecol27: 287-291. [Crossref]
- Vaidyanathan S, van Velzen D, Krishnan KR, Parsons KF, Soni BM, et al. (1996) Nerve fibres in urothelium and submucosa of neuropathic urinary bladder: an immunohistochemical study with S-100 and neurofilament.Paraplegia34: 137-151. [Crossref]
- Lundeberg T, Liedberg H, Nordling L, Theodorsson E, Owzarski A, et al. (1993) Interstitial cystitis: correlation with nerve fibres, mast cells and histamine.Br J Urol71: 427-429. [Crossref]
- Christmas TJ, Rode J, Chapple CR, Milroy EJ, Turner-Warwick RT (1990) Nerve fibre proliferation in interstitial cystitis.Virchows Arch A Pathol Anat Histopathol416: 447-451. [Crossref]
- Akbar A, Yiangou Y, Facer P, Brydon WG, Walters JR, et al. (2010) Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain.Gut59: 767-774. [Crossref]
- Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, et al. (2008) Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain.Gut57: 923-929. [Crossref]
- Moore KH, Gilpin SA, Dixon JS, Richmond DH, Sutherst JR (1992) Increase in presumptive sensory nerves of the urinary bladder in idiopathic detrusor instability.Br J Urol70: 370-372. [Crossref]
- Mills IW, Greenland JE, McMurray G, McCoy R, Ho KM, et al. (2000) Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation.J Urol163: 646-651. [Crossref]
- Weström LV, Willén R (1998) Vestibular nerve fiber proliferation in vulvar vestibulitis syndrome.Obstet Gynecol91: 572-576. [Crossref]
- Bohm-Starke N, Hilliges M, Falconer C, Rylander E (1998) Increased intraepithelial innervation in women with vulvar vestibulitis syndrome.Gynecol Obstet Invest46: 256-260. [Crossref]
- Signorile PG, Campioni M, Vincenzi B, D'Avino A, Baldi A (2009) Rectovaginal septum endometriosis: an immunohistochemical analysis of 62 cases.In Vivo23: 459-464. [Crossref]
- Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, et al. (2000) Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod 15: 1744-1750.
- Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, et al. (2000) Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod 15: 1744-1750.
- Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, et al. (2002) Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod 17: 1895-1900.
- Anaf V, Chapron C, El Nakadi I, De Moor V, Simonart T, et al. (2006) Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis.Fertil Steril86: 1336-1343. [Crossref]
- Mechsner S, Schwarz J, Thode J, Loddenkemper C, Salomon DS, et al. (2007) Growth-associated protein 43-positive sensory nerve fibers accompanied by immature vessels are located in or near peritoneal endometriotic lesions.Fertil Steril88: 581-587. [Crossref]
- Zhu L, Huang Q, Huang X, Zhang J, Xu H, et al. (2014) Decreased nerve fibers in the oviduct isthmus of women with endometriosis.Acta Histochem116: 871-877. [Crossref]
- Donnez O, Soares M, Defrčre S, Dehoux JP, van Langendonckt A, et al. (2013) Nerve fiber density in deep nodular endometriotic lesions induced in a baboon experimental model. Fertil Steril 100:1144-1150.
- Yan D, Liu X, Guo SW (2017) Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis.Eur J Obstet Gynecol Reprod Biol209: 14-24. [Crossref]
- Mechsner S, Kaiser A, Kopf A, Gericke C, Ebert A, et al. (2009) A pilot study to evaluate the clinical relevance of endometriosis-associated nerve fibers inperitoneal endometriotic lesions. Fertil Steril 92: 1856-1861.
- Barcena de Arellano ML, Oldeweme J, Arnold J, Schneider A, Mechsner S (2013) Remodeling of estrogen-dependent sympathetic nerve fibers seems to be disturbed in adenomyosis.Fertil Steril100: 801-809. [Crossref]
- Lee R (1840) Memoirs on the anatomy and ganglia of the nerves of the uterus. London.
- Lee R (1978) On the nervous ganglia of the uterus, and an appendix to a paper on the nervous ganglia of the uterus, with a further account of the nervous structures of that organ. Phil Trans of the Royal Society of London, part I, pp. 269-275, 1841, and part II, pp. 173-179, 1842. Am J Obstet Gynecol. 131: 217-218.
- Manuel DE (2001) Robert Lee, the uterine nervous system and a wrangle at the Royal Society 1839-1849.J R Soc Med94: 645-647. [Crossref]
- Spackman R, Wrigley B, Roberts A, Quinn MJ (2007) The anatomy of the inferior hypogastric plexus: a different view. J Obstet Gynaecol 27: 130-133.
- Mitchell GA (1938) The Innervation of the Ovary, Uterine Tube, Testis and Epididymis.J Anat72: 508-517. [Crossref]
- Cervero F (1996) Spinal cord mechanisms of hyperalgesia and allodynia: role of peripheral input from nociceptors.Prog Brain Res113: 413-422. [Crossref]
- Golding J (2004) ALSPAC Study Team. The Avon Longitudinal Study of Parents and Children (ALSPAC)--study design and collaborative opportunities. Eur J Endocrinol 151 Suppl 3: U119-23.
- Farland LV, Eliassen AH, Tamimi RM, Spiegelman D, Michels KB, et al. (2017) History of breast feeding and risk of incident endometriosis: prospective cohort study. BMJ 2017 358: j3778.
- Lane WA (1924) A Clinical lecture on chronic intestinal stasis; or, our mechanical relationship to our surroundings.Br Med J1: 142-144. [Crossref]
- Burkitt DP (1973) Some diseases characteristic of modern Western civilization.Br Med J1: 274-278. [Crossref]
- Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, et al. (1992) Defecation frequency and timing, and stool form in the general population: a prospective study.Gut33: 818-824. [Crossref]
- Heaton KW, Cripps HA (1993) Straining at stool and laxative taking in an English population.Dig Dis Sci38: 1004-1008. [Crossref]
- Wu XQ, Xia WT, Cai YY, Quinn MJ (2016) Medical evacuation of the uterus and subsequent preterm labor.Am J Obstet Gynecol215: 809-810. [Crossref]
- Quinn MJ (2016) Re: A systematic review and network meta-analysis comparing the use of Foley catheters, misoprostol and dinoprostone for cervical ripening in induction of labour: Excessive, uterine activity is a maternal, neurological emergency.BJOG123: 2048. [Crossref]
- Li YY, Gu QR, Chen GR, Quinn MJ (2018) Arteriolar Injury in Hypertension.Am J Med131: e133-133e134. [Crossref]
- Quinn MJ (2017) The Etiology of Arteriolar Injury in Malignant Hypertension.Am J Hypertens30: e13-13e14. [Crossref]
- Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, et al. (2016) Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes.Am J Obstet Gynecol215: 484. [Crossref]
- SavitskiÄ GA, Morozov VV, Svechnikova FA, Ivanova RD (1981) [Pathogenesis of uterine myoma development].Akush Ginekol (Mosk): 13-15. [Crossref]
- SavitskiÄ GA, Skopichev VG, Rakitskaia VV (1986) ["Denervation" of the tumor node as one of the elements of the pathogenesis of uterine myoma].Akush Ginekol (Mosk): 24-27. [Crossref]
- SavitskiÄ GA, Skopichev VG, Shelest VN (1987) [The pathogenesis of uterine myomas].Vestn Akad Med Nauk SSSR: 62-68. [Crossref]
- Wu XQ, Cai YY, Xia WT, Quinn MJ (2016) The aetiology of pre-eclampsia, 1945-1953.BJOG123: 2130. [Crossref]
- Chaban VV (2008) Visceral sensory neurons that innervate both uterus and colon express nociceptive TRPV1 and P2X3 receptors in rats. Ethn Dis 18(2 Suppl 2): S2-20-4.
- Franklin KJ (1962) The utero-renal reflex: the haemex phenomenon.J Obstet Gynaecol Br Emp69: 506-507. [Crossref]
- Xia WT, Cai YY, Wang BQ, Wu XQ, Quinn MJ (2016) Injuries to uterotubal nerves in advanced painless adenomyosis. Presented at International Society for Reproductive Surgery and the Fallopian Tube. Royal College of Obstetricians & Gynaecologists.
- Cullen TS (1908) The pathogenesis of adenomyoma. WB Saunders.
- Quinn M (2009) Endometriosis: the elusive epiphenomenon.J Obstet Gynaecol29: 590-593. [Crossref]
- Burnstock G (2009) Purinergic mechanosensory transduction and visceral pain.Mol Pain5: 69. [Crossref]
- Roberge S, Bujold E, Nicolaides KH (2018) Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis.Am J Obstet Gynecol218: 287-293. [Crossref]



