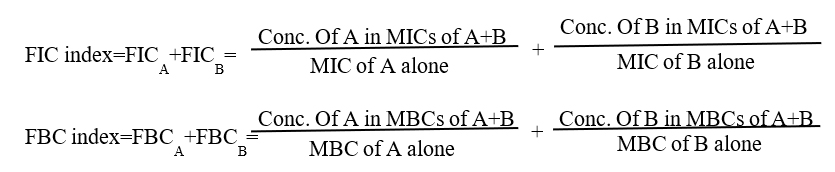Selaginella tamariscina (S. tamariscina) (Beauv.) Spring (Selaginellaceae) has been used in oriental medicine for the treatment of dysmenorrhea, chronic hepatitis, hyperglycemia, amenorrhea, hematuria, prolapse of the anus and metrorrhagia. This study aimed to investigate the synergistic antibacterial activity with existing antimicrobial agents against oral pathogen. The synergistic effects of 50% ethanol extract of S. tamariscina (STE) were evaluated against oral bacteria, either alone or with antibiotics, via broth microdilution and time-kill method. The minimal inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) values for STE, ampicillin and gentamicin against all the tested bacteria ranged between 6.25-50/50-200 μg/mL, 0.0625-16/0.25-32 μg/mL, and 4-128/16-256 μg/mL, respectively. STE in combination with ampicillin showed a strong synergistic effect against oral bacteria (fractional inhibitory concentration (FIC) index ≤0.5), whereas on combining with gentamicin, it reduced the on half-eighth times than used alone (FICI ≤ 0.5). Furthermore, a time-kill study showed that the growth of the tested bacteria was completely attenuated after 2-6 h of treatment with 1/2 MIC of STE with 1/2 MIC of antibiotics resulted from an increase of the rate of killing in units of CFU/mL to a greater degree than was observed with alone. The results of this study demonstrate the antimicrobial and synergistic activity of STE and antibiotics against oral pathogens.
Selaginella tamariscina, antibacterial activity, oral pathogen, synergistic effect, minimum inhibitory concentrations (MICs), minimum bactericidal concentrations (MBCs)
STE: The ethanol extract of Selaginella tamariscina; MICs: Minimum inhibitory concentrations; MBCs: Minimum bactericidal concentrations; CFU: Colony forming unit; FIC index: Fractional inhibitory concentration; FBC index: Fractional bactericidal concentration index
Oral health problems, particularly periodontal diseases, dental caries and endodontic infections, are the most significant destructive processes in the oral cavity and are a costly burden to the public globally [1-3]. Dental caries (tooth decay or cavities) are the most common and widespread chronic oral diseases that affect children and adults [4]. They are irreversible infectious diseases of the teeth leading to cavities in the teeth structure, thus compromising the structure and function of the teeth [5]. Until a few decades ago, development of caries was ascribed to a few gram-positive bacterial species in the biofilm, i.e., the specific biofilm/plaque hypothesis, and Streptococcus mutans, Streptococcus sobrinus together with some Lactobacillus species were regarded as key pathogens [6,7]. Periodontal diseases are pathologic conditions of bacterial infection of the structures around the teeth (including the gums, the cementum that covers the root, the periodontal ligament and the alveolar bone) that can lead to tooth loss affecting more than half of all adults [8,9]. Generally, the etiological agents of periodontal diseases are Gram-negative rods including Aggregatibacter actinomycetemcomitans, Tannerella forsythia, Prevotella, Fusobacterium, and Porphyromonas gingivalis [9,10]. Recent reports have suggested a potential role for periodontal infections in more serious systemic diseases including cardiovascular disease, respiratory infections, and diabetes, which are pathologies that significantly affect the overall health of the infected individual [11].
Mechanical dental plaque removal is an efficient procedure to prevent periodontitis and caries [12]. However, the use of chemical compounds as a complementary method is also necessary and has proven to be a valuable tool to decrease tooth biofilm formation [13,14]. Selaginella tamariscina (S. tamariscina) (Beauv.) Spring (Selaginellaceae) has been used in oriental medicine for the treatment of dysmenorrhea, chronic hepatitis, hyperglycemia, cancer, amenorrhea, hematuria, prolapse of the anus, and metrorrhagia [15-17]. Pharmacological studies on S. tamariscina have reported its anti-inflammatory, antibacterial, anti-hypertensive and anti-hyperglycemic activities [17-20]. The investigation of the phytochemical constituents of S. tamariscina revealed it to be an abundant source of biflavonoids such as amentoflavone, hinokiflavone, isocryptomerine, sotetsuflavone, and sumaflavone [18,21]. The biflavonoids isolated from S. tamariscina are known to display a variety of biological activities involving anti-inflammatory, anti-allergic, antitumor, antioxidant, antidiabetic, antiviral, and anticancer activities, and osteogenesis [15,18,20,21].
In this study, the antimicrobial activities of 50% ethanol extract of Selaginella tamariscina (STE) against oral bacteria were assessed using broth microdilution method and time-kill method for synergistic effect of the combination with antibiotics.
Plant material and preparation of 50% ethanol extract of Selaginella tamariscina (STE)
Dried Selaginella tamariscina (P.Beauv.) Spring (Selaginella Herba, known in Korea as Kwon Baek or Boo Cheo Son) (100g ) was macerated and were extracted in 8-fold volumes of 50% ethanol (800 mL) at 80°C for 4 h. The extract was then filtered, concentrated using a rotary vacuum evaporator (EYELA, Japan), lyophilized using a freeze dryer, and stored at 4°C. The yield of the lyophilized extract obtained was 18.5% (w/w) of dried S. tamariscina.
Bacterial strains
The oral bacterial strains used in this study were: Streptococcus mutans ATCC 25175 (American Type Culture Collection), Streptococcus sanguinis ATCC 10556, Streptococcus parasanguinis KCOM 1497 (Korean Collection for Oral Microbiology), Streptococcus sobrinus ATCC 27607, Streptococcus ratti KCTC (Korean Collection for type cultures) 3294, Streptococcus criceti KCTC 3292, Streptococcus downei KCOM 1165, Streptococcus anginosus ATCC 31412, Streptococcus gordonii ATCC 10558, Aggregatibacter actinomycetemcomitans ATCC 43717, Fusobacterium nucleatum ATCC 10953, Prevotella intermedia ATCC 25611, and Porphylomonas gingivalis ATCC 33277. Brain-Heart Infusion (Difco Laboratories, Detroit, MI) broth supplemented with 1% yeast extract (Difco) was used for all bacterial strains except P. intermedia and P. gingivalis. For P. intermedia and P. gingivalis, BHI broth containing hemin 1 μg/mL (Sigma) and menadione 1 μg/mL (Sigma) was used.
Minimum inhibitory concentrations/minimum bactericidal concentrations assay
The minimum inhibitory concentrations (MICs) were determined for 50% ethanol extract of S. tamariscina (STE) by the broth dilution method, and were carried out in triplicate. The antibacterial activities were examined after incubation at 37℃ for 18 h (facultative anaerobic bacteria), for 24 h (microaerophilic bacteria), and for 1-2 days (obligate anaerobic bacteria) under anaerobic conditions, used a mix of H2 and nitrogen (N2) (5/95%) or N2/carbon dioxide (CO2)/H2 (85/10/5 %) to remove oxygen. MICs were determined as the lowest concentration of test samples that resulted in a complete inhibition of visible growth in the broth. MIC50s, defined as MICs at which, 50% of MIC of oral bacteria were inhibited, were determined. Following anaerobic incubation of MICs plates, the minimum bactericidal concentrations (MBCs) were determined on the basis of the lowest concentration of STE that kills 99.9% of the test bacteria by plating out onto each appropriate agar plate. Ampicillin and gentamicin (Sigma) were used as standard antibiotics in order to compare the sensitivity of STE against oral bacteria.
Checkerboard dilution test
The antibacterial effects of a combination of STE and antibiotics were assessed by the checkerboard test as previously described [22,23]. The antimicrobial combinations assayed included STE with antibiotics, ampicillin, gentamicin, erythromycin, and vancomycin. Serial dilutions of two different antimicrobial agents were mixed in cation-supplemented Mueller-Hinton broth. After 24-48 h of incubation at 37°C, the MICs were determined to be the minimal concentration at which there was no visible growth and MBCs were determined on the basis of the lowest concentration of STE that kills 99.9% of the test bacteria by plating out onto each appropriate agar plate. The fractional inhibitory concentration (FIC)/ fractional bactericidal concentration (FBC) index was calculated according to the equation:
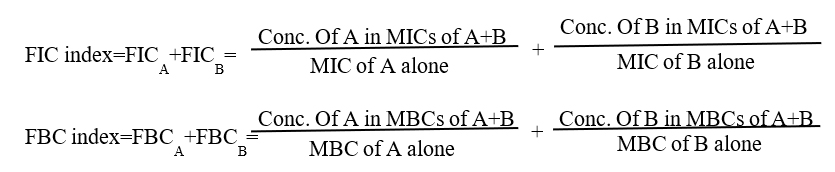
The FIC and FBC index are the sum of the FICs and FBCs of each of the drugs, which in turn is defined as the MIC and MBC of each drug when it is used in combination divided by the MIC and MBC of the drug when it is used alone. The interaction was defined as synergistic if the FIC and FBC index was less than or equal to 0.5, additive if the FIC and FBC index was greater than 0.5 and less than or equal 1.0, indifferent if the FIC and FBC index was greater than 1.0 and less than or equal to 2.0, and antagonistic if the FIC and FBC index was greater than 2.0 [22].
Time-kill and growth inhibition curves assay
Bactericidal activities of STE and antibiotics under study were also evaluated using time-kill curves on oral bacteria. Tubes containing Mueller-Hinton supplemented to which antibiotics had been added at concentrations of the 1/2 MIC were inoculated with a suspension of the test strain, giving a final bacterial count between 5~7×106 CFU/mL. The tubes were thereafter incubated at 37°C in an anaerobic chamber and viable counts were performed at 0, 0.5, 1, 2, 3, 4, 5, 6, 12 and 24 h after addition of antimicrobial agents, on agar plates incubated for up to 48 h in anaerobic chamber at 37°C. Antibiotic carryover was minimized by washings by centrifugation and serial 10-fold dilution in sterile phosphate-buffered saline, pH 7.3. Colony counts were performed in duplicate, and means were taken. The solid media used for colony counts were BHI agar for streptococci and BHI agar containing hemin and menadione for P. intermedia and P. gingivalis.
Minimum inhibitory concentrations/minimum bactericidal concentrations of STE and antibiotics
The use of natural products and herbal medicines has been documented in the past. They have been reported to be effective in the management of many infections in general. Some of these have been assessed in the recent past for their antimicrobial potential against oral bacteria [24-26]. STE was evaluated for their antimicrobial activities against thirteen oral bacterial species present in the oral cavity. The results of the antimicrobial activity showed that STE exhibited antimicrobial activities against cariogenic bacteria at MICs, 6.25 to 50 μg/mL; MBCs, 25 to 100 μg/mL, against periodontopathogenic bacteria at MICs, 12.5 to 50 μg/mL; MBCs, 50 to 200 μg/mL and for ampicillin, either MIC/MBCs 0.0625/0.25 or 16/32 μg/mL; for gentamicin, either MIC/MBCs 4/16 or 128/256 μg/mL on tested all bacteria (Table 1). The MIC50 and MIC90 ranges of STE were from 3.13 to 12.5 μg/mL and 12.5 to 50 μg/mL, respectively. The STE showed stronger antimicrobial activity against S. mutans, S. criceti, S. gordonii, and P. gingivalis at MIC/MBC, 6.25/25-12.5/50 μg/mL than another bacteria at MIC/MBC, 25-50/50-200 μg/mL. Isocryptomerin isolated from Selaginella tamariscina showed potent antibacterial activity against gram-positive and gram-negative bacterial strains including clinical isolates of multidrug-resistant bacteria [19,27].
Table 1. Antibacterial activity of Selaginella tamariscina ethanol extract (STE) and antibiotics in oral bacteria
Samples |
STE (μg/mL) |
Ampicillin |
Gentamicin |
MIC50< |
MIC90< |
MIC/MBC |
MIC/MBC (μg/mL) |
S. mutans
ATCC 251751 |
3.13 |
12.5 |
12.5/50 |
0.25/0.5 |
4/16 |
S. sanguinis
ATCC 10556 |
3.13 |
25 |
25/100 |
0.125/0.5 |
8/32 |
S. sobrinus
ATCC 27607 |
12.5 |
25 |
25/50 |
0.125/0.5 |
16/32 |
S. ratti
KCTC 32942 |
12.5 |
50 |
50/100 |
0.25/1 |
16/32 |
S. criceti
KCTC 3292 |
3.13 |
12.5 |
12.5/50 |
0.0625/0.25 |
8/32 |
S. anginosus
ATCC 31412 |
12.5 |
50 |
50/100 |
0.25/1 |
8/16 |
S. gordonii
ATCC 10558 |
1.56 |
6.25 |
6.25/25 |
0.125/0.5 |
16/64 |
A. actinomycetemcomitans
ATCC 43717 |
12.5 |
50 |
50/100 |
16/32 |
4/16 |
F. nucleatum
ATCC 51190 |
25 |
50 |
50/200 |
8/16 |
4/16 |
P. intermedia
ATCC 49049 |
6.25 |
25 |
25/50 |
0.5/1 |
32/64 |
P. gingivalis
ATCC 33277 |
3.13 |
12.5 |
12.5/50 |
0.5/2 |
128/256 |
1American Type Culture Collection (ATCC)
2Korean collection for type cultures (KCTC)
Synergistic effect of STE with antibiotics
Many antimicrobial preparations, such as conventional antibiotics, chlorhexidine (CHX), phenolic compounds and triclosan, can inhibit bacteria biofilm effectively [28-30]. However, extensive use of these antimicrobial agents can lead to some side-effects, such as tooth staining, calculus formation, drug resistance and gastrointestinal reactions [30,31]. Therefore, searching for new antimicrobial molecules, which exhibit few or no side-effects and long-term retention in oral cavity, has been intensified in recent years [32]. The synergistic effects of STE with antibiotics or with antibiotics were evaluated in oral bacteria (Tables 2 and 3). In combination with ampicillin, STE was reduced ≥4-8 fold in all tested bacteria, except S. sobrinus, S. criceti, and P. intermedia, producing a synergistic effect as defined by FICI ≤ 0.5. The MBC for ampicillin was shown synergistic effects in S. criceti, F. nucleatum, P. intermedia, and P. gingivalis by FBCI ≤ 0.5 (Table 2). In combination with STE, the MIC for gentamicin was reduced ≥4-8-fold in all tested bacteria, except S. sobrinus by FICI ≤ 0.5 and MBC except S. sobrinus, S. anginosus, and P. intermedia by FBCI ≤ 0.75-1 (Table 3). Amentoflavone (AF) is a biflavonoid compound extracted from S. tamariscina Spring. as a representative biflavonoid with several pharmacological functions, the bioavailability of AF with intraperitoneal injection was 77.4%±28.0%. AF has pharmacological activities including anti-inflammatory, anti-microbial, and anti-oxidative [15,18,19].
Table 2. Synergistic effects of Selaginella tamariscina ethanol extract (STE) with ampicillin against oral bacteria
Strains |
Agent |
MIC/MBC (μg/mL) |
FIC/FBC |
FICI/FBCI2 |
Outcome |
Alone |
Combination1 |
S. mutans
ATCC 251753 |
STE |
12.5/50 |
3.13/12.5 |
0.25/0.25 |
0.5/0.75 |
Synergistic/Additive |
Ampicillin |
0.25/0.5 |
0.0625/0.25 |
0.25/0.5 |
S. sanguinis
ATCC 10556 |
STE |
25/100 |
6.25/25 |
0.25/0.25 |
0.5/0.75 |
Synergistic/Additive |
Ampicillin |
0.125/0.5 |
0.0313/0.25 |
0.25/0.5 |
S. sobrinus
ATCC 27607 |
STE |
25/50 |
12.5/25 |
0.5/0.5 |
0.75/0.75 |
Additive/Additive |
Ampicillin |
0.125/0.5 |
0.0313/0.125 |
0.25/0.25 |
S. ratti
KCTC 32944 |
STE |
50/100 |
12.5/25 |
0.25/0.25 |
0.5/0.5 |
Synergistic/Synergistic |
Ampicillin |
0.25/1 |
0.0313/0.25 |
0.25/0.25 |
S. criceti
KCTC 3292 |
STE |
12.5/50 |
3.13/12.5 |
0.25/0.25 |
0.75/0.5 |
Additive/Synergistic |
Ampicillin |
0.0625/0.25 |
0.0313/0.0625 |
0.5/0.25 |
S. anginosus
ATCC 31412 |
STE |
50/100 |
12.5/50 |
0.25/0.5 |
0.5/0.75 |
Synergistic/Additive |
Ampicillin |
0.25/1 |
0.0625/0.25 |
0.25/0.25 |
S. gordonii
ATCC 10558 |
STE |
6.25/25 |
1.56/12.5 |
0.25/0.5 |
0.5/0.75 |
Synergistic/Additive |
Ampicillin |
0.125/0.5 |
0.0313/0.125 |
0.25/0.25 |
A. actinomycetemcomitans
ATCC 43717 |
STE |
50/100 |
12.5/25 |
0.25/0.25 |
0.5/0.75 |
Synergistic/Additive |
Ampicillin |
16/32 |
4/16 |
0.25/0.5 |
F. nucleatum
ATCC 51190 |
STE |
50/200 |
12.5/50 |
0.25/0.25 |
0.5/0.5 |
Synergistic/Synergistic |
Ampicillin |
8/16 |
2/4 |
0.25/0.25 |
P. intermedia
ATCC 49049 |
STE |
25/50 |
12.5/12.5 |
0.5/0.25 |
0.75/0.5 |
Additive/Synergistic |
Ampicillin |
0.5/1 |
0.125/0.25 |
0.25/0.25 |
P. gingivalis
ATCC 33277 |
STE |
12.5/50 |
3.13/12.5 |
0.25/0.25 |
0.375/0.5 |
Synergistic/Synergistic |
Ampicillin |
0.5/2 |
0.0625/0.5 |
0.125/0.25 |
1The MIC and MBC of the Selaginella tamariscina ethanol extract (STE) with ampicillin
2The fractional inhibitory concentration (FIC) index/fractional bactericical concentration (FBC) index
3American Type Culture Collection (ATCC)
4Korean collection for type cultures (KCTC)
Table 3. Synergistic effects of Selaginella tamariscina ethanol extract (STE) with gentamicin against oral bacteria
Strains |
Agent |
MIC/MBC (μg/mL) |
FIC/FBC |
FICI/FBCI2 |
Outcome |
Alone |
Combination1 |
S. mutans
ATCC 251753 |
STE |
12.5/50 |
3.13/12.5 |
0.25/0.25 |
0.5/0.5 |
Synergistic/Synergistic |
Gentamicin |
4/16 |
1/4 |
0.25/0.25 |
S. sanguinis
ATCC 10556 |
STE |
25/100 |
6.25/25 |
0.25/0.25 |
0.5/0.5 |
Synergistic/Synergistic |
Gentamicin |
8/32 |
2/8 |
0.25/0.25 |
S. sobrinus
ATCC 27607 |
STE |
25/50 |
6.25/25 |
0.25/0.5 |
0.75/1 |
Additive/Additive |
Gentamicin |
16/32 |
8/16 |
0.5/0.5 |
S. ratti
KCTC 32944 |
STE |
50/100 |
12.5/25 |
0.25/0.25 |
0.5/0.5 |
Synergistic/Synergistic |
Gentamicin |
16/32 |
4/8 |
0.25/0.25 |
S. criceti
KCTC 3292 |
STE |
12.5/50 |
3.13/6.25 |
0.25/0.125 |
0.5/0.25 |
Synergistic/Synergistic |
Gentamicin |
8/32 |
2/4 |
0.25/0.125 |
S. anginosus
ATCC 31412 |
STE |
50/100 |
12.5/25 |
0.25/0.25 |
0.5/0.75 |
Synergistic/Additive |
Gentamicin |
8/16 |
2/8 |
0.25/0.5 |
S. gordonii
ATCC 10558 |
STE |
6.25/25 |
1.56/6.25 |
0.25/0.25 |
0.5/0.375 |
Synergistic/Synergistic |
Gentamicin |
16/64 |
4/8 |
0.25/0.125 |
A. actinomycetemcomitans
ATCC 43717 |
STE |
50/100 |
12.5/25 |
0.25/0.25 |
0.5/0.375 |
Synergistic/Synergistic |
Gentamicin |
4/16 |
1/2 |
0.25/0.125 |
F. nucleatum
ATCC 51190 |
STE |
50/200 |
12.5/50 |
0.25/0.25 |
0.5/0.375 |
Synergistic/Synergistic |
Gentamicin |
4/16 |
1/2 |
0.25/0.125 |
P. intermedia
ATCC 25611 |
STE |
25/50 |
6.25/25 |
0.25/0.5 |
0.5/0.625 |
Synergistic/Additive |
Gentamicin |
32/64 |
8/8 |
0.25/0.125 |
P. gingivalis
ATCC 33277 |
STE |
12.5/50 |
3.13/12.5 |
0.25/0.25 |
0.5/0.5 |
Synergistic/Synergistic |
Gentamicin |
128/256 |
32/64 |
0.25/0.25 |
1The MIC and MBC of the Selaginella tamariscina ethanol extract (STE) with gentamicin
2The fractional inhibitory concentration (FIC) index/fractional bactericical concentration (FBC) index
3American Type Culture Collection (ATCC)
4Korean collection for type cultures (KCTC)
Time kill of STE with antibiotics
Previous studies had reported the antimicrobial activities and mechanisms of STE against several kinds of bacteria [19,27]. However, the type of microorganisms and their cell membrane structure and composition could play an important role in the susceptibility to antimicrobials [33,34]. Overcome drug-resistance mechanisms are the use of combination of antibiotics, such as β-lactams together with β-lactamase inhibitors. β-lactam antibiotics are known to inhibit the synthesis of the bacterial cell wall by binding to the reactive Ser62 of the D-alanyl-D-alanine carboxypeptidase/transpeptidase, which catalyzes the final step in the cross-linking of the bacterial cell wall peptidoglycan [35,36]. The MIC value was similar to the results that MIC values of STE against Staphylococcus aureus and Candida albicans were 20 μg/mL and 50 μg/mL, respectively [27]. The bacterial effect of STE with antibiotics, ampicillin and gentamicin against oral bacteria was confirmed by time-kill curve experiments. The STE (MIC or 1/2 MIC) alone resulted rate of killing increasing or not changing in CFU/ml at time dependent manner, with a more rapid rate of killing by STE (1/2 MIC) with ampicillin or/and gentamicin (1/2 MIC) (Figures 1 and 2). A strong bactericidal effect was exerted in drug combinations.
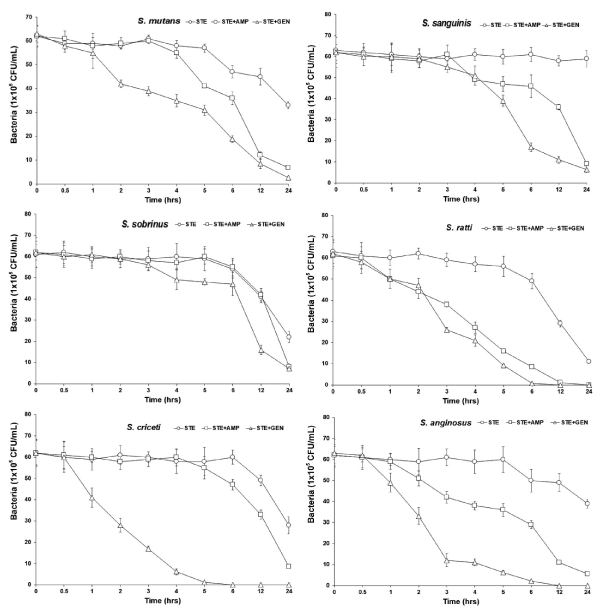
Figure 1. Time-kill curves of MIC of STE alone and its combination with 1/2 MIC of AMP and GEN against S. mutans, S. sanguinis, S. sobrinus, S. ratti, S. criceti, and S. anginosus. Bacteria were incubated with STE (○), STE + AMP (□), and STE + GEN (△) over time. CFU, colony-forming units
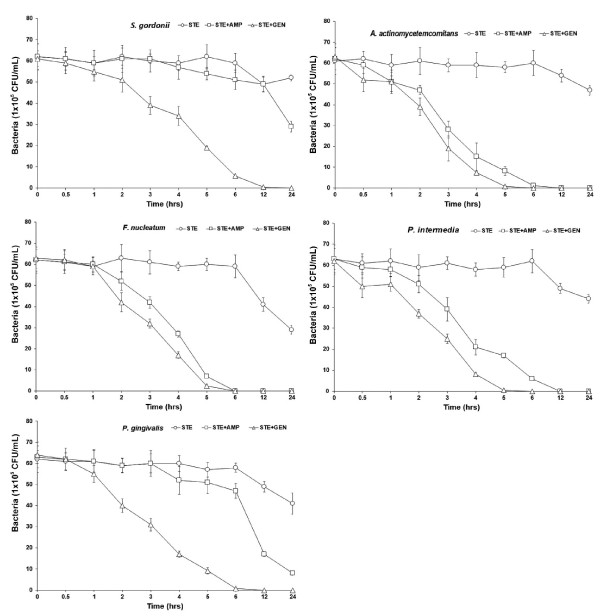
Figure 2. Time-kill curves of MIC of STE alone and its combination with 1/2 MIC of AMP and GEN against S. gordonii, A. actinomycetemcomitans, F. nucleatum, P. intermedia, and P. gingivalis. Bacteria were incubated with STE (○), STE + AMP (□), and STE + GEN (△) over time. CFU, colony-forming units
In conclusion, these findings suggest that crude ethanol extract of S. tamariscina exhibited a wide range of pharmacological effects establish the conditions required of a novel cariogenic bacteria and periodontal pathogens, particularly bacteroides species drug and may be useful in the future in the treatment of oral bacteria biofilm.
The authors declare no conflict of interest.
This study was not funded by any organization.
This study has no need for prior approval by an ethics committee.
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, et al. (2010) The human oral microbiom. J Bacteriol 192: 5002-5017.
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43: 5721-5732.
- Grossner-Schreiber B, Fetter T, Hedderich J, Kocher T, Schreiber S, et al. (2006) Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: a case-control study. J Clin Periodontol 33: 478-484.
- Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, et al. (2017) Dental caries. Nat Rev Dis Primers 3: 17030.
- de Soet JJ (2015) Dissertation 25 years after date 42. Dental caries and the role of specific bacteria. Ned tijdschr tandheelkd 122: 525-531.
- Do T, Devine DA, Marsh PD (2013) Oral biofilms: molecular analysis, challenges, and future prospects in dental diagnostics. Clin Cosmet Investig Dent 5: 11-9.
- Larsen T, Fiehn NE (2017) Dental biofilm infections-an update. APMIS 125: 376-384.
- Lagdive SS, Lagdive SB, Mani A, Anarthe R, Pendyala G, et al. (2013) Correlation of mast cells in periodontal disease. J Indian Soc Periodontol 17: 63-67.
- Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis primers 3: 17038.
- Ardila CM, López MA, Guzmán IC (2011) Positive correlations between presence of gram negative enteric rods and Porphyromonas gingivalis in subgingival plaque. Acta Odontol Latinoam 24: 15-19.
- Nazir MA (2017) Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci 11: 72-80.
- Ramakrishna Y, Goda H, Baliga MS, Munshi AK (2011) Decreasing cariogenic bacteria with a natural, alternative prevention therapy utilizing phytochemistry (plant extracts). J Clin Pediatr Dent 36: 55-63.
- Jeong SI, Kim BS, Keum KS, Lee KH, Kang SY, et al. (2013) Kaurenoic acid from Aralia continentalis inhibits biofilm formation of Streptococcus mutans. Evid Based Complement Alternat Med 2013: 160592.
- O’Toole GA (2011) Microtiter dish biofilm formation assay. J Vis Exp 2011: e2437.
- Jung YJ, Lee EH, Lee CG, Rhee KJ, Jung WS, et al. (2017) AKR1B10-inhibitory Selaginella tamariscina extract and amentoflavone decrease the growth of A549 human lung cancer cells in vitro and in vivo. J Ethnopharmacol 202: 78-84.
- Wang CG, Yao WN, Zhang B, Hua J, Liang D, et al. (2018) Lung cancer and matrix metalloproteinases inhibitors of polyphenols from Selaginella tamariscina with suppression activity of migration. Bioorg Med Chem Lett 28: 2413-2417.
- Zheng XK, Li YJ, Zhang L, Feng WS, Zhang X (2011) Antihyperglycemic activity of Selaginella tamariscina (Beauv.) Spring. J Ethnopharmacol 133: 531-537.
- Shim SY, Lee SG, Lee M. (2018) Biflavonoids Isolated from Selaginella tamariscina and Their Anti-Inflammatory Activities via ERK 1/2 Signaling. Molecules 23: E926.
- Hwang JH, Choi H, Woo ER, Lee DG (2013) Antibacterial effect of amentoflavone and its synergistic effects with antibiotics. J Microbiol Biotechnol 23: 953-958.
- Zheng XK, Wang WW, Zhang L, Su CF, Wu YY, et al. (2013) Antihyperlipidaemic and antioxidant effect of the total flavonoids in Selaginella tamariscina (Beauv.) Spring in diabetic mice. J Pharm Pharmacol 65: 757-766.
- Zhu QF, Shao LD, Wu XD, Liu JX, Zhao QS (2019) Ioslation, structural assignment of Isoselagintamarlin A from Selaginella tamariscina and its biomimetic synthesis. Nat Prod Bioprospect 9: 69-74.
- Cha JD, Jeong MR, Jeong SI, Lee KY (2007) Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens. J Microbiol Biotechnol 17: 858-864.
- Ma XH, Zheng CJ, Han LY, Xie B, Jia J, et al. (2009) Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov Today 14: 579-588.
- Khalid M, Hassani D, Bilal M, Butt ZA, Hamayun M, et al. (2017) Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia Arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch Oral Biol 81: 175-185.
- Choi HA, Cheong DE, Lim HD, Kim WH, Ham MH, et al. (2017) Antimicrobial and Anti-Biofilm Activities of the Methanol Extracts of Medicinal Plants against Dental Pathogens Streptococcus mutans and Candida albicans. J Microbiol Biotechnology 27: 1242-1248.
- Ferrazzano GF, Roberto L, Catania MR, Chiaviello A, De Natale A, et al. (2013) Screening and scoring of antimicrobial and biological activities of Italian vulerary plants against major oral pathogenic bacteria. Evid Based Complement Alternat Med 2013: 316280.
- Lee J, Choi Y, Woo ER, Lee DG (2009) Antibacterial and synergistic activity of isocryptomerin isolated from Selaginella tamariscina. J Microbiol Biotechnol 19: 204-207.
- Pandit S, Kim HJ, Park SH, Jeon JG (2012) Enhancement of fluoride activity against Streptococcus mutans biofilms by a substance separated from Polygonum cuspidatum. Biofouling 28: 279-287.
- Van Strydonck DA, Slot DE, Van der Velden U, Van der Weijden F (2012) Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol 39: 1042-1055.
- Baehni PC, Takeuchi Y (2003) Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis 9: 23-29.
- Bhardwaj P, Krishnappa S (2014) Various approaches for prevention of dental caries with emphasis on Probiotics: A review. IOSR J Dent Med Sci 1: 62-67.
- Wang HY, Cheng JW, Yu HY, Lin L, Chih YH, Pan YP (2015) Efficacy of a novel antimicrobial peptide against periodontal pathogens in both planktonic and polymicrobial biofilm states. Acta biomater 25, 150-161.
- Nowotarska SW, Nowotarski KJ, Friedman M, Situ C (2014) Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers. Molecules 19: 7497-7515.
- Roces C, Rodríguez A, Martínez B (2012) Cell wall-active bacteriocins and their applications beyond antibiotic activity. Probiotics Antimicrob Proteins 4: 259-272.
- Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15: 639-652.
- Kuzin AP, Liu H, Kelly JA, Knox JR (1995) Binding of cephalothin and cefotaxime to D-ala-D-ala-peptidase reveals a functional basis of a natural mutation in a low-affinity penicillinbinding protein and in extended-spectrum beta-lactamases. Biochemistry 34: 9532-9540.