Abstract
The development of photosensitizers and light sources has enabled the use of antimicrobial photodynamic therapy (aPDT) in various dental therapies. In the present study, we compared the antibacterial and cytotoxic effects of Au clusters photoexcited by blue and white LED irradiation. We fabricated novel photosensitizers, captopril-protected gold (Capt-Au) clusters and lysozyme-stabilized gold (Lyz-Au) clusters, for aPDT. Au clusters were then photoexcited by two kinds of light sources, blue high-power and white low-power light-emitting diodes (LEDs). Since white LED contains a wide spectrum of light (400–750 nm), white LED would be relevant for aPDT even if using a low-power source.
The turbidity and viability of Streptococcus mutans were assessed following application of Capt-Au clusters (500 μg/mL) or Lyz-Au clusters (1,000 μg/mL) photoexcited by a blue high-power LED (1,000 mW/cm2) or white low-power LED (80 mW/cm2). In addition, the cytotoxicity of Au clusters and LED irradiation was evaluated in NIH3T3 and MC3T3-E1 cells.
Au clusters photoexcited by the white low-power LED equally decreased the turbidity and viability of S. mutans compared with blue high-power LED. However, Au clusters photoexcited by white LED irradiation caused decreased cytotoxicity in mammalian cells compared with those photoexcited by blue LED irradiation. In conclusion, white LEDs possess biosafe properties for aPDT using Au clusters.
Key words
Antimicrobial photodynamic therapy, captopril-protected gold (Au) clusters, lysozyme-stabilized gold clusters, photosensitizer, Streptococcus mutans
Introduction
Antimicrobial photodynamic therapy (aPDT) has been extensively investigated for the treatment of various infections by bacteria, fungi and viruses [1-3]. A novel photosensitizer, gold (Au) cluster, has the ability to generate singlet oxygen (1O2) via photoexcitation [4]. Miyata et al. [5]. reported that 25 Au atoms and 18 captopril (Capt) ligands as the protector, Capt-Au clusters, decreased the growth of oral bacteria, such as Streptococcus mutans, Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis, after irradiation with a blue light-emitting diode (LED) of dental curing light. Hence, Au clusters would be useful for prevention and treatment of dental caries and periodontal disease through aPDT.
Since Capt-Au clusters have strong absorbance in the range of 300–500 nm with a peak at 450 nm, blue LED irradiation is suitable for photoexcitation of Au clusters [6]. However, blue LEDs have short wavelengths and produce a higher amount of energy, which can lead to adverse biological effects. Irradiation with a blue high-power LED exhibits phototoxic effects [7], thus low-power LED irradiation would be desirable for biosafety in the clinical use of aPDT. Kawasaki et al. [4]. reported that Au clusters had a broad light absorption spectrum and efficiently generated 1O2 under visible/near-infrared (532, 650 and 808 nm) irradiation. As white LEDs include a wide range of wavelengths (400–750 nm), a white LED would efficiently photoexcite Au clusters even under low-power irradiation. Accordingly, we speculated that white low-power LED light is suitable for a biosafe device for photoexcitation of Au clusters.
In this study, we fabricated two types of Au clusters, Capt-Au clusters and lysozyme-stabilized gold (Lyz-Au) clusters. We then assessed the antimicrobial and cytotoxic effects of Au clusters photoexcited with two light devices, blue high-power and white low-power LEDs.
Materials and methods
Synthesis of Au clusters
Capt-Au clusters were synthesized according to a previously described method [4,8]. Tetrachloroauric (III) acid (0.20 mmol, Wako Pure Chemical Industries, Ltd., Osaka, Japan) and tetraoctylammonium bromide (0.23 mmol, Wako Pure Chemical Industries, Ltd.) were dissolved in 10 mL methanol. Subsequently, captopril (1 mmol, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was injected into the reaction mixture and stirred for 30 min. Sodium borohydride (2 mmol) was added to the mixture and stirred for 12 h at room temperature. The resultant mixture was centrifuged to remove insoluble Au(I) polymer. The supernatant was collected and concentrated by rotary evaporation and then Capt-Au clusters were precipitated by ethanol and dried in a vacuum.
The synthesis of Lyz-Au clusters was performed according to a previously described method [9]. In brief, 5 mL of HAuCl4 solution (10 mM) was thoroughly mixed with 5 mL of Lyz solution (50 mg/mL Lysozyme, from Egg White, Wako Pure Chemical Industries, Ltd.) under vigorous stirring at 300 rpm for 3 min. Thereafter, 1 mL of 1M NaOH solution was added to the mixed solution. After reaction at 37°C for 6 h, the solution containing Lyz-Au clusters was purified using a centrifugal ultrafiltration tube (Amicon® Ultra, Merck Ltd., Tokyo, Japan)
Preparation of bacterial suspension
S. mutans strain ATCC 35668 was kept frozen until analysis. Bacterial stocks were incubated in brain heart infusion (BHI) broth (Pearlcore®, Eiken Chemical, Co., Ltd., Tokyo, Japan) supplemented with 0.1% antibiotics (gramicidin D and bacitracin, Wako Pure Chemical Industries, Ltd.) and 1% sucrose.
Thermometry after LED irradiation
BHI medium (500 μL) was dispensed into 48-well microplates and irradiated by a blue high-power LED light device at a wavelength of 420–460 nm (1,000 mW/cm2, PenCure, Morita Corporation, Tokyo, Japan) or white low-power LED light device at a wavelength of 420–750 nm (80 mW/cm2, SPF-D2, Shodensha, Osaka, Japan). The temperature of the medium was measured at 20-s intervals for a total of 100 s using an infrared radiation thermometer (AD-5617, A&D, Co., Ltd., Tokyo, Japan).
Antibacterial effects of Au clusters photoexcited by LEDs
To adjust the gold content of the solution, diluted Capt-Au clusters (final concentration, 500 µg/mL) and Lyz-Au clusters (final concentration, 1,000 µg/mL) were added to the suspension containing S. mutans [final concentration, 5.5×106 colony-forming units (CFU)/mL]. Subsequently, the suspension was dispensed into a microplate and irradiated by blue or white LED light for 1 min before anaerobic incubation at 37°C. As a control, a suspension not containing Au clusters was assessed.
After incubation for 24 h, the turbidity of the suspension was measured using a turbidimeter (CO7500 Colourwave, Funakoshi Co., Ltd, Tokyo, Japan) at an absorbance of 590 nm. In addition, the viability of S. mutans was assessed using WST-8 (Cell Counting Kit-8, Dojindo Laboratories, Mashiki, Japan) in accordance with the manufacturer’s instructions. The absorbance was measured using a microplate reader (ETY-300, Toyo Sokki, Yokohama, Japan) at 450 nm.
Cytotoxic effects of Au clusters photoexcited by LEDs
To evaluate cytotoxicity, 1×104 mouse fibroblastic NIH3T3 cells (RIKEN BioResource Center, Tsukuba, Japan) and osteoblastic MC3T3-E1 cells (RIKEN BioResource Center) were grown in 96-well plates using culture medium (MEM-alpha, GlutaMAX-I, Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Qualified, Thermo Fisher Scientific) and 1% antibiotics (Pen Strep, Thermo Fisher Scientific). Capt-Au clusters (500 µg/mL) or Lyz-Au clusters (1,000 µg/mL) were added to the medium. Before incubation, the suspension was irradiated with blue high-power or white low-power LED light for 1 min. The cultures were then incubated at 37°C with 5% CO2. The Au clusters-containing medium exchange and subsequent LED irradiation for 1 min were performed every 2 days. As a control, a suspension not containing Au clusters was assessed.
The cytotoxicity after 2, 4, 6 and 8 days of incubation was evaluated using the WST-8 assay and lactate dehydrogenase (LDH) assay (Cytotoxicity LDH Assay Kit-WST, Dojindo Laboratories) following the manufacturer’s instructions. The absorbance at 450 nm (WST-8) and 490 nm (LDH) was measured on a microplate reader.
Statistical analysis
Statistical analysis was performed by Scheffé’s test. 𝑃 values < 0.05 were considered statistically significant. All statistical procedures were performed using a software package (SPSS 11.0, IBM Corporation, Armonk, NY, USA).
2021 Copyright OAT. All rights reserv
Results
Thermometry after LED irradiation
Blue high-power LED irradiation remarkably increased the temperature of the medium from 24°C to 46°C. Specimen temperature following 60 s of blue LED irradiation was approximately 36°C. Conversely, white low-power LED irradiation did not change the temperature of the medium.
Antibacterial effects of Au clusters and LEDs on S. mutans
Figure 1A and 1B show the turbidity and viability of S. mutans, respectively. Application of Au clusters resulted in a significant reduction of bacterial turbidity and viability (P<0.05). The reduction of turbidity and viability by white low-power LED irradiation was comparable to that of blue high-power LED irradiation. No significant differences were observed between Capt-Au and Lyz-Au clusters regarding the turbidity of S. mutans. However, Lys-Au clusters decreased the viability of S. mutans compared with Capt-Au clusters (P<0.05).
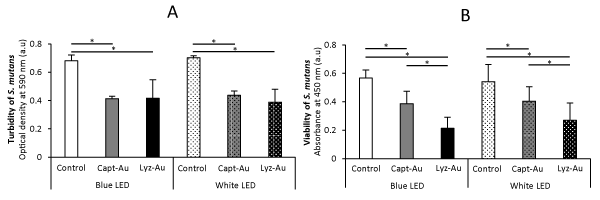
Fig. 1: Turbidity and viability of S. mutans after 24-h incubation (n=6, mean ± SD).
(A) Turbidity of S. mutans. (B) Viability of S. mutans. *, P<0.05.
Cytotoxic effects of Au clusters and LEDs on mammalian cells
The results of WST-8 assays are presented in Figure 2. At 2 days, all cells equivalently proliferated regardless of the application of Au clusters and LED irradiation. However, cell viability at 4, 6 and 8 days was decreased by repetitive applications of Au clusters and LED irradiation compared with control. In control NIH3T3 cells, blue high-power LED irradiation significantly suppressed cell viability compared to white low-power LED irradiation at all time points (P<0.05). In addition, applying blue LED irradiation decreased the 8-day viability of cells compared with white LED irradiation.
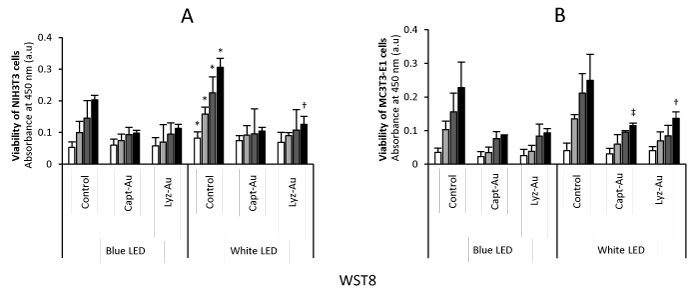
Fig. 2. Viability of NIH3T3 cells and MC3T3-E1 cells at 2, 4, 6 and 8 days (n=6, mean ± SD)
(A) Viability of NIH3T3 cells. (B) Viability of MC3T3-E1 cells. *, P<0.05 vs. control after blue LED irradiation; †, P<0.05 vs. Lyz-Au clusters after blue LED irradiation; ‡, P<0.05 vs. Capt-Au clusters after blue LED irradiation.
The results of LDH assays are presented in Figure 3. Regardless of Au cluster application, blue high-power LED irradiation tended to enhance the LDH activity of mammalian cells compared with white low-power LED irradiation.
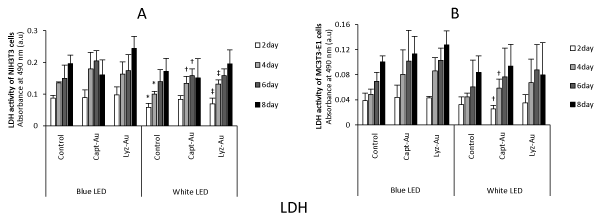
Fig. 3. LDH activity of NIH3T3 cells and MC3T3-E1 cells at 2, 4, 6 and 8 days (n=6, mean ± SD)
(A) Viability of NIH3T3 cells. (B) Viability of MC3T3-E1 cells. *, P<0.05 vs. control after blue LED irradiation; †, P<0.05 vs. Capt-Au clusters after blue LED irradiation; ‡, P<0.05 vs. Lyz-Au clusters after blue LED irradiation.
Discussion
PDT requires a light source that activates the photosensitizer. Various light sources, such as tungsten filament, quartz halogen, xenon arc, metal halide and phosphor-coated sodium lamps, are available for PDT [1-3]. Recently, LEDs have been applied in PDT for oral cancer and infection therapies [10,11]. The advantages of LEDs include small size, highly flexible and low cost in comparison with conventional lasers.
In this study, the turbidity and viability of S. mutans were reduced by photoexcited Au clusters, suggesting that 1O2 generated by Au clusters attacked S. mutans. In addition, the suppressive effects of Au clusters photoexcited by blue high-power and white low-power LEDs were comparable. We speculate that Au clusters were adequately photoexcited by the white low-power LED to obtain antibacterial effects against S. mutans. Multiple wavelengths within the white LED spectrum can individually photoexcite Au clusters, leading to explosive 1O2 generation from Au clusters, even under low-power irradiation. Conversely, blue LEDs have a narrow spectrum, therefore, antibacterial 1O2 generation of Au clusters requires high-power photoexcitation of blue LEDs.
The assessment of cytotoxicity revealed that blue high-power LED irradiation decreased cell viability and increased LDH production in mammalian cells. These findings suggested that the blue high-power LED exhibited cytotoxicity, regardless of Au cluster application. The blue LED has a short wavelength and produces a higher amount of energy. Long-term irradiation with a blue high-power LED may cause thermal injury. Indeed, a blue LED has been reported to increase the production of reactive oxygen species, causing oxidative stress to biological cells as compared with a white LED [7]. The phototoxic effects of blue high-power LED irradiation may result in the failure of tissue healing following aPDT.
Conclusion
We assessed the antimicrobial and cytotoxic effects of Au clusters photoexcited by blue high-power or white low-power LED irradiation. The suppression of bacterial cell growth by white LED irradiation was comparable to that of blue LED irradiation. In addition, cytotoxicity of the white LED in mammalian cells was lower than that of the blue LED. Therefore, white low-power LEDs possess biosafe properties and would be suitable for aPDT using Au clusters.
Acknowledgments
This work was supported by JSPS KAKENHI (Grant Nos. JP15H03520, JP15H03526, JP26505011, JP26107719 and JP16K11822) and Hitachi Metals Materials Science Foundation. A part of this work was supported by the Nanotechnology Platform Program (Molecule and Material Synthesis) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
- Konopka K, Goslinski T (2007) Photodynamic therapy in dentistry. J Dent Res 86: 694-707. [Crossref]
- Wilson M (2004) Lethal photosensitization of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci 3: 412-418. [Crossref]
- Rajesh S, Koshi E, Philip K, Mohan A (2011) Antimicrobial photodynamic therapy: An overview. J Indian Soc Periodontol 15: 323-327. [Crossref]
- Kawasaki H, Kumar S, Li G, Zeng C, Kauffman DR, et al (2014) Generation of singlet oxygen by photoexcited Au25(SR)18 Clusters. Chem Mater 26: 2777-2778.
- Miyata S, Miyaji H, Kawasaki H, Yamamoto M, Nishida E, et al (2017) Antimicrobial photodynamic activity and cytocompatibility of Au25(Capt)18 clusters photoexcited by blue LED light irradiation. Int J Nanomed 12: 2703-2716. [Crossref]
- Kumar S, Jin R (2012) Water-soluble Au25(Capt)18 nanoclusters: synthesis, thermal stability, and optical properties. Nanoscale 4: 4222-4227. [Crossref]
- Kuse Y, Ogawa K, Tsuruma K, Shimazawa M, Hara H (2014) Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci Rep 4: 5223. [Crossref]
- Hirakawa K (2009) Fluorometry of singlet oxygen generated via a photosensitized reaction using folic acid and methotrexate. Anal Bioanal Chem 393: 999-1005. [Crossref]
- Russell BA, Jachimska B, Komorek P, Mulheran PA, Chen Y (2017) Lysozyme encapsulated gold nanoclusters: effects of cluster synthesis on natural protein characteristics. Phys Chem Chem Phys 19, 7228-7235. [Crossref]
- Lim HJ, Oh CH (2011) Initial clinical experiences with the photodynamic therapy (PDT) of oral cavity carcinomas. Photodiagnosis Photodyn Ther 8: 337-42.
- Costa AC1, Campos Rasteiro VM, da Silva Hashimoto ES, Araújo CF, Pereira CA, et al. (2012) Effect of erythrosine- and LED-mediated photodynamic therapy on buccal candidiasis infection of immunosuppressed mice and Candida albicans adherence to buccal epithelial cells. Oral Surg Oral Med Oral Pathol Oral Radiol 114: 67-74. [Crossref]



