Introduction: Grave’s disease is the most common cause of hyperthyroidism. Methimazole (MMI), radioactive iodine (RAI) therapy and surgery are currently the available therapies for Graves’s disease. Common side effects post RAI therapy include hypothyroidism and transient hyperthyroidism. Cerebrovascular accident (CVA) as a result of post RAI thyrotoxicosis is however a less reported side effect.
Case: 75 year old female with history of Grave’s disease presented to our clinic with difficult to control hyperthyroidism on MMI as her thyroid function tests (TFTs) were fluctuating between hypo and hyperthyroidism (TSH from 0.01 to 51 uU/ml) and MMI dose was being adjusted every 1-2 months. She was treated with RAI therapy and received 15 millicuries dose of I-131. She continued MMI 30 mg daily until 5 days before RAI and restarted MMI same dose the next day and continued propranolol 80 mg twice daily. Three days post RAI, she presented to ER with right sided facial droop and slurred speech. On physical examination, she had tachycardia with irregularly irregular pulse, right facial weakness and flattening of right nasolabial fold. Complete blood count and complete metabolic panel were unremarkable. TFTs were consistent with post RAI thyrotoxicosis with TSH 0.090 uU/ml, free T4 2.42 ng/dl and free T3 6.08 pg/ml. CT angiogram of head and neck revealed acute infarction in the left insula. MRI Brain also showed acute infarction in the cortex and white matter of the left insular lobe. EKG showed atrial fibrillation. NIH stroke scale was 4 but tissue plasminogen activator (tPA) was not given due to guaiac positive stool. She was treated with epixaban, rosuvastatin and sotalol. Patient was finally diagnosed with acute CVA due to cardioembolic phenomenon in the setting of post RAI thyrotoxic exacerbation. She was discharged home with outpatient neurology and endocrine follow up. Her dysarthria continued to improve with no further focal deficits. Six weeks later, TFTs also improved with TSH 31.04 uU/mL and free T4 0.78 ng/dl and was started on low dose levothyroxine.
Discussion: This case described a patient with Graves’ disease, who suffered atrial fibrillation and cardioembolic CVA in the setting of post RAI thyrotoxicosis. Thyrotoxicosis due to RAI in patients with hyperthyroidism is known complication but can be life threatening. This case highlights the rare complications like atrial fibrillation and stroke in post RAI thyrotoxicosis despite the appropriate use of MMI and Beta adrenergic blocker. Patients and treating physicians should be aware of this rare entity before procedure.
Graves’ disease, radioactive iodine therapy, hyperthyroidism
Hyperthyroidism is characterized by increased thyroid hormone synthesis and secretion from the thyroid gland [1]. Graves’ disease (GD) is the most common cause of hyperthyroidism in all age groups in United States [2]. Hyperthyroidism is more common in women than in men and lifetime risk is 3% for women and 0.5% for men [2]. Treatment options for GD include antithyroid medications (ATMs), surgery or radioactive iodine (RAI). Side effects of RAI treatment are transient hyperthyroidism, hypothyroidism, thyroid swelling, sialadenitis, immunogenic effect, carcinogenicity, and teratogenicity. Absolute contraindications to RAI are pregnancy, lactation, planning pregnancy, moderate to severe opthalmopathy, inability to comply with radiation safety regulation [3]. We are presenting a rare case of stroke associated with post RAI thyrotoxicosis.
75 year old female with GD on methimazole (MMI) for two years presented to clinic. Thyroid function tests (TFTs) kept fluctuating between hypo and hyperthyroidism (TSH ranged from less than 0.01 - 51 uU/mL) on MMI and dose was adjusted every 1-2 months. On presentation, TSH was 0.109 uU/mL (0.270-4.200 uU/mL), free T4 1.78 ng/dl (0.90-1.70 ng/dl), and free T3 3.52 pg/ml (2.00-4.40 pg/ml). Thyroid stimulating immunoglobulin 493% (< 122%) was high. Thyroid uptake scan showed increased homogenous uptake of 66% and 68.4% at 3 and 25 hours, respectively. Because of difficulty controlling hormone levels, she was offered RAI therapy and received 15 millicuries. She restarted MMI 30 mg daily next day post RAI after holding it for 5 days before treatment and continued propranolol twice daily. Three days post RAI treatment; she presented to ER with right sided facial droop, slurred speech and was tachycardic with irregularly irregular pulse, right facial weakness with flattening of right nasolabial fold. Routine blood work was unremarkable. TFTs were consistent with post RAI thyrotoxicosis with TSH 0.090 uU/mL, free T4 2.42 ng/dl, free T3 6.08 pg/ml. CT scan of head was negative. MRI Brain showed acute infarction in the left insular lobe (Figure 1). Electrocardiogram showed atrial fibrillation (AF). Echocardiogram showed mild concentric hypertrophy with left ventricular ejection fraction 40%. NIH stroke scale was 4 and despite patient been in window period, tissue Plasminogen activator was not administered due to history of melena and guaiac positive stool. She was treated with apixaban, rosuvastatin and sotalol. There were no obvious other risk factors for AF and stroke. She was diagnosed with acute stroke due to cardioembolic phenomenon in the setting of post RAI thyrotoxic exacerbation and discharged home with speech therapy, neurology and endocrine follow up. Dysarthria gradually improved and was slowly tapered off MMI. Six weeks later, TFT were TSH 31.04 uU/mL and free T4 0.78 ng/dl, and started on levothyroxine.
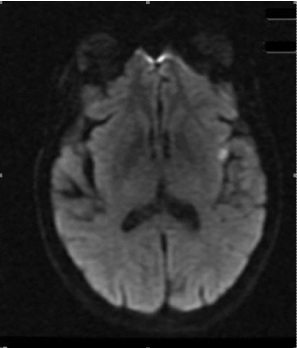
Figure 1. Acute infarction in left insular lobe.
Incidence of thyrotoxicosis post RAI is known but is very rare and has been reported to be 0.4% [4]. However, AF with stroke after RAI therapy outside the thyroid storm has not been reported to the best of our knowledge. Severe exacerbation of thyrotoxic symptoms or thyroid storm post RAI is due to injury to thyroid cells caused by I-131, releasing increased amount of stored thyroid hormones into the circulation [5]. Lee et al., [4] had hypothesized an immunological exacerbation as a possible cause of persistent and severe hyperthyroidism after RAI therapy in GD. High level of TSH-receptor antibodies at diagnosis can be associated with a post therapy flare up of thyrotoxicosis [6]. Patients with GD who are at increased risk of complications due to worsening of hyperthyroidism include those who are extremely symptomatic, have thyroid hormone level 2-3 times the upper limit of normal, the elderly, and those with substantial comorbidities especially cardiovascular disease [6]. Such patients should be treated with either beta-adrenergic blocker and / or ATM prior to RAI therapy [6]. However, the frequency of short-term worsening of hyperthyroidism following RAI treatment in patients who have received pretreatment with ATM is not known. The comorbidities which increases the risk of cardiovascular complications includes underlying AF, heart failure, pulmonary hypertension, renal failure, infection, trauma, poorly controlled diabetes mellitus, cerebrovascular or pulmonary disease [7]. Medical therapy for these comorbid conditions should be optimized prior to administering RAI treatment [8]. In addition, beta-adrenergic blocking drugs should be used judiciously in such high risk patients in preparation for RAI therapy [7]. If given as pretreatment, ATM should be discontinued 3-5 days before the RAI therapy, restarted 3-7 days later, and generally tapered over 4-6 weeks as thyroid function normalizes. Patients also should be made aware of these rare but potentially detrimental side effects and should have close follow up by endocrinologists [4].
Thyrotoxicosis exacerbation with complications like AF and stroke post RAI in hyperthyroid patients are rare but can be life threatening. Pretreatment evaluation for risk factors and appropriate use of ATM, beta adrenergic blockers and close follow up can help to reduce occurrence of these events. Awareness among physicians in the community regarding this is vital.
Source(s) of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
- De Leo S, Lee SY, Braverman LE (2016) Hyperthyroidism. Lancet 388: 906-918. [Crossref]
- Smith TJ, Hegedüs L (2016) Graves' Disease. N Engl J Med 375: 1552-1565. [Crossref]
- Read CH, Tansey MJ, Menda Y (2004) A 36-year retrospective analysis of the efficacy and safety of radioactive iodine in treating young graves' patients. J Clin Endocrinol Metab 89: 4229-4233. [Crossref]
- Lee SW, Lee J, Bae JH, Seo JH, Kang SM, et al. (2009) Paradoxical exacerbation of preexisting Graves' disease induced by insufficient radioiodine treatment: A report of five patients. Nucl Med Commun 30: 275-280. [Crossref]
- Shafer RB, Nuttall FQ (1971) Thyroid crisis induced by radioactive iodine. J Nucl Med 12: 262-264. [Crossref]
- Bonnema SJ, Hegedus L (2012) Radioiodine therapy in benign thyroid diseases: Effects, side effects, and factors affecting therapeutic outcome. Endocr Rev 33: 920-980. [Crossref]
- McDermott MT, Kidd GS, Dodson LE, Hofeldt FD (1983) Radioiodine-induced thyroid storm. Case report and literature review. Am J Med 75: 353-359. [Crossref]
- Vijayakumar V, Nusynowwitz ML, Ali S (2006) Is it safe to treat hyperthyroid patients with I-131 without fear of thyroid storm? Ann Nucl Med 20: 383-385. [Crossref]

