Abstract
Introduction: Symptomatic relief of occasional or transitorily recurrent oral ulcers, often severely painful, is paramount. Counteracting the oral tissue loss would likewise be beneficial. After enzymatic cleavage, natural-origin polynucleotides (PNs) may help alleviate the oral tissue’s ulcerative losses by passively replenishing the indispensable pool of small nitrogen precursors (nucleosides, nucleotides, nitrogen bases) and making it readily available in the oral mucosa. PNs demonstrated these properties in other conditions as facilitating the healing of torpid wounds. Moreover, PNs show persistent moisturizing and lenitive properties and synergise with hyaluronic acid (HA).
Methods: A questionnaire-based survey in a prospective cohort of 104 subjects with aphthae and other recurrent ulcers of the oral mucosa after topical application of a mucoadhesive PNs/HA-based gel (CE0373 medical device) to evaluate the device’s persisting efficacy and safety on oral pain and discomfort.
Results: The mean total symptom scores before and after administration of the oral care device improved from 2.5 ± 0.92 at baseline to 1.3 ± 0.52 at final follow-up (p<0.001). Mean final oral pain scores never exceeded 1.4 for all symptoms, much less than the moderate severity threshold, with improvements most remarkable for generalised oral pain (from 3.0 ± 1.07 at baseline to 1.4 ± 0.72, p<0.001) and discomfort and pain discomfort due to chewing (from 3.0 ± 1.00 at baseline to 1.3 ± 0.63, p<0.001) and irritating foods (from 3.0 ± 1.10 at baseline to 1.2 ± 0.48, p<0.001).
Conclusions: The real-world monitoring survey demonstrated that the PNs/HA-based oral care gel retains its long-standing record of efficient performance and excellent safety with no variations over time.
Key words
aphthae, oral ulcerations, oral protective gel, polynucleotides, stomatitis.
Introduction
The clinical history is variable: for instance, it may be months of severe ulcers of the oral and oropharyngeal mucosa lasting 7 to 10 days, with new ulcers arising every few days and troubling difficulties in swallowing. On in-depth questioning, the evidence of small blisters, soon bursting and leaving ulcers severely painful due to the rich innervation of oral mucosae, is the clue to the diagnosis. Histology, histochemistry, and serum antibodies to the epithelial basement membrane confirm the diagnosis of pemphigus vulgaris [1].
Conversely, it may be a long history since the youth of crops of two to five round to oval recurrent, painful, non-indurated, less than 10-mm aphthous ulcers usually healing in the third life decade. The yellow-white or yellow-grey pseudo-membrane sloughing base, the inflammatory erythematous halo, and the prodromal burning sensation up to 48 hours before the ulcer’s development suggest recurrent aphthous stomatitis (RAS), often repeatedly occurring in families with an up to 90% chance for children with RAS-positive parents to develop the disorder [1,2]. Often associated with haematinic nutritional deficiencies (up to 20% of patients are deficient in iron, folic acid or vitamin B12) and coeliac disease (up to 20% of patients) or other immune-inflammatory bowel disorders, even clinically mild, RAS ulcers spontaneously heal within two weeks. Ulcers often exacerbate during school or university examinations, revealing the role of stress [1-3].
Accidental trauma, overextended dentures, or sharp broken teeth may also cause a transient oral ulcer; the same with some food additives like cochineal red, azorubine and amaranth; even some drugs like aspirin causing caustic burns, and nicorandil and are bisphosphonates associated with fixed and lichenoid drug eruptions. Surgical sequelae may also manifest as persistent painful ulcers [2-4].
Shortly, the range of occasional or transitorily recurrent oral ulcers is wide. The possibility of an underlying systemic condition that may account for the ulceration further complicates the differential diagnosis and therapy [1,2]. Dermatoses like erosive and atrophic lichen planus, discoid and systemic lupus erythematosus, erythema multiforme and linear IgA disease are prominent examples [1,2].
Occasional or transitorily recurrent oral ulcers, including RAS, usually heal within two weeks without scarring and may benefit from symptomatic relief with over-the-counter medications during the active erosive phase [2]. Topical corticosteroids in carboxymethylcellulose paste and antimicrobial mouthwashes, e.g., chlorhexidine digluconate, povidone-iodine or benzydamine hydrochloride mouth rinses, mitigate painful symptoms yet hardly reduce recurrences [2].
Oral ulcer treatments should accelerate healing and extend the ulcer-free period [2]. Natural-origin polynucleotides (PNs) are an enticing alternative topical option to other adhesive or mouthwash treatments. Highly purified from fish tissues, PNs, in combination with biotechnological sodium hyaluronate (HA) in a mucoadhesive gel, are available as a Class III CE0373 medical device. The polyvinylpyrrolidone (PVP)-based formulation ensures the mucoadhesive properties crucial for wound repair.
After adhesion to the oral mucosa, a protective film develops, acting as a barrier against contact between oral mucosae and the tongue, saliva, and solid and liquid food. In the meantime, the hydrophilic PNs undergo enzymatic cleavage and progressively release a steady flow of nucleosides, nucleotides and nitrogen bases that diffuse in the superficial layers of oral mucosae, thereby increasing their tissue pools [5].
PNs properties, including the moisturizing and viscoelastic effects, have been exploited for years in various dermatology and plastic surgery indications to improve hydration, turgidity and skin elasticity and promote the healing of chronic wounds and ulcers [6-9]. Injectables medical devices combining PNs and HA proved beneficial in aesthetic medicine and treating vulvovaginal atrophy symptoms [10,11].
The paper reports the outcomes of a real-world survey study centred on a questionnaire distributed to individuals with persistent or recurrent oral ulcers of variable aetiology, including post-surgical erosive sequelae. The investigation was the first in a long-term program to monitor the clinical performance and safety of the PNs/HA medical device. Confirming the profile of known side effects and contraindications and identifying any unknown side effects or emergent risks was another purpose of the study and the long-term monitoring program.
Materials and methods
Exploratory, experimental design: prospective study cohort of 104 individuals of both genders, including children, with oral discomfort due to persistently painful recurrent oral ulcers by variable causes, including oral surgery, enrolled in the same oral care centre to prevent site bias. Before the survey, all individuals had undergone a topical treatment with a Class III CE0373 PNs/HA-based gel medical device (NUCLIASKIN® Oral Care, Mastelli S.r.l.) containing 1% PNs (about 150 mg out of the 15 grams of the formulation) and 1.5% HA (about 220 mg in the 15-g medical device). Table 1 illustrates the cohort demographics and the distribution of aetiologies among surveyed subjects. All subjects applied the gel device according to approved indications: aphthae, erosions and ulcers due to dental prosthesis and orthodontic devices, and wounds secondary to surgical procedures (Table 1).
Table 1. Demographics of the interviewed individuals treated with the mucoadhesive PNs/HA-based device before the survey (upper table) and distribution of ulcer aetiologies (lower table)
Demographics of cohort individuals (*)
Age |
Mean ± SD (years old) |
50.0 ± 16.97 |
Range (years old) |
10 to 80 |
Median (years old) |
53 |
Gender |
Female (n, %) |
60 (57.7%) |
Male (n, %) |
44 (42.3%) |
(*) Data from 101 subjects out of 104
Aetiologies (**)
|
Individuals |
Per cent |
Lesions after oral surgery |
61 |
58.7 |
Lesions after trauma or other causes |
9 |
8.7 |
Canker sores |
12 |
11.5 |
Prosthesis lesions |
7 |
6.7 |
Irritation after hygiene and prophylaxis treatment |
19 |
18.3 |
Other aetiologies |
4 |
3.8 |
(**) Total: 112subjects with multiple answers allowed (per cent total higher than 100%)
The preliminary interventional phase called for the oral application of the reparative gel until the healing of erosions. Fifty-two per cent of the cohort individuals applied the oral care device three times per day (mean 3.2 ± 0.62) (Table 2).
Table 2. Number and per cent distribution of topical gel applications by interviewed subjects per day
Daily frequency of gel device applications on the oral mucosa
Applications per day |
Individuals |
% |
2 |
11 |
10.6 |
3 |
52 |
50.0 |
4 |
25 |
24.0 |
More than 4 |
16 |
15.4 |
The survey took advantage of an anonymous paper questionnaire prospectively offered to eligible patients with oral lesions by the investigators. The survey phase followed the preliminary interventional phase. The survey aimed to confirm the performance of the gel device shown by previous studies and clinical experience in the general population:accelerated ulcer healing, symptom suppression, and high safety profile. The surveyed symptoms were generalized oral pain and discomfort, pain and discomfort when chewing, pain and discomfort on contact with the tongue, pain and discomfort when talking, irritation while eating salty, spicy or acidic foods and pain and discomfort when swallowing. The survey also aimed to identify any unknown adverse event and any never previously detected emergent risk.
A systematic review of the literature helped to develop the five-point, six-closed question questionnaire designed to provide a rapid assessment of how, in the subject’s opinion, oral pain, discomfort, and irritation had evolved since baseline.
The survey questionnaire administered to the surveyed individuals after the preliminary interventional phase is available at the end of the paper as supplementary material.
Persisting efficacy on symptoms
The surveyed individuals rated the severity of known symptoms on a Likert-scale developed explicitly for the survey. For each surveyed individual, the symptom severity scores ranged from 1 (no symptom) to 5 (extreme severity), and the final total symptom scores from 6 to 30. A question also asked for the time to symptom resolution for assessing if the surveyed oral care device effectively accelerates healing to less than the usual seven to fourteen days.
The reason to use five-point Likert scales instead of 10-point scales was that outcomes assessed on five-point scales have unimodal and symmetric distributions while 10-point scales have highly skewed J- and U-shaped distributions. Outcomes assessed on five-point scales also have lower means and floor and ceiling effects. At the same time, regression analysis shows that such scales account for a significant fraction of total variance in both floor and ceiling effects and minimize the contribution of unaccounted factors [12].
Safety assessment
Based on spontaneous reporting by cohort subjects (open questions in the questionnaire), to identify known side effects, describe their clinical presentation and severity, and identify any previously unknown adverse event or emergent risk. The adverse event observations by the investigator complemented the individual spontaneous reports.
Statistics
The sample size was estimated with the G*Power statistical program version 3.14 based on the worst-case hypothesis and considering two effect sizes. The sample size calculation was performed considering the total symptom score (whole population) and assuming a conservative 65% improvement after topical application of the PNs/HA-based device due to the lack of specific literature [13]. Descriptive statistics included median values and 95% confidence intervals.
The inferential statistical analysis compared the mean total severity scores for symptoms before and after treatment with the oral care device, using the paired two-sample Student’s t-test for within-subject variations (aka dependent sample t-test) to assess whether the mean difference between two sets of observations is zero [14]. All statistical tests were two-sided with a 5% significance level. Statistical analysis program: StatPlus release v7 [15].
Results
The improvement in the total symptom scores before and after administration of the oral care gel device was highly significant (Figure 1): on average, from 2.5 ± 0.92 at baseline to 1.3 ± 0.52 (p<0.001), with almost all patients symptom-free at the end of treatment.
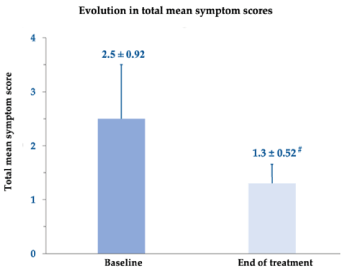
Figure 1.Changes in total mean symptom scores between baseline and end of treatment. # p <0.001
All the surveyed symptoms significantly improved after treatment with the oral gel in most subjects. Figure 2 illustrates the mean score variations for generalized oral pain and discomfort, pain and discomfort when chewing, pain and discomfort on contact with the tongue, pain and discomfort while talking, irritation when eating salty, spicy or acidic foods, and pain and discomfort when swallowing: all scores showed either significant or highly significant improvements.
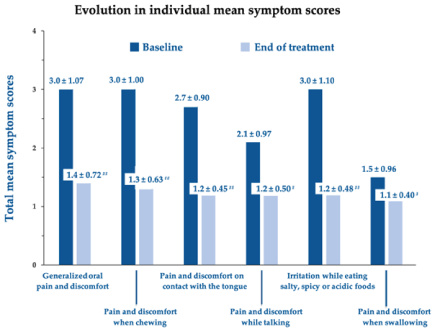
Figure 2.Changes in individual mean symptom scores between baseline and end of treatment. # p <0.001, ## p <0.0001
More in detail, Figures 3 and 4 illustrate, respectively, how the impact of generalised oral pain and discomfort and pain and discomfort when chewing decreased between baseline and end of treatment in the surveyed individuals. All surveyed subjects suffered from generalised pain and discomfort before treatment, 31.4% of them severely, while pain disappeared with the oral care device in 68.6% of subjects at follow-up; all residual pain and discomfort (reported by only 2% of the cohort subjects) were mild or moderate and never severe. Likewise, pain and discomfort when chewing, unreported in only 12.6% of surveyed individuals at baseline, missed in up to 73.7% of subjects after treatment, while pain and discomfort due to chewing (in almost nine subjects out of ten at baseline independently of severity) persisted in only 26.3% of surveyed subjects at the end-of-treatment follow-up.
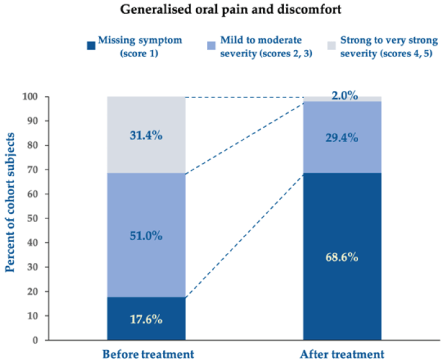
Figure 3.Score changes in generalised oral pain and discomfort between baseline before treatment and follow-up after treatment. Chi-square test for per cent distributions: p <0.001
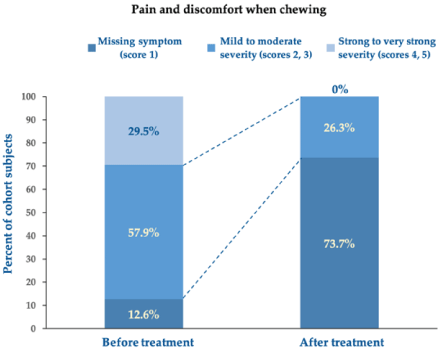
Figure 4Score changes in pain and discomfort when chewing between baseline before treatment and follow-up after treatment. Chi-square test for per cent distributions: p <0.001
In the opinion of surveyed subjects, the control of symptoms was rapid-on average, 3.7 ± 2.09 days, with 15.4% of the cohort subjects reporting symptom relief within one day, more than two-thirds (68.3%) within three days, and less than a third (31.7%) within four or more days (Figure 5).
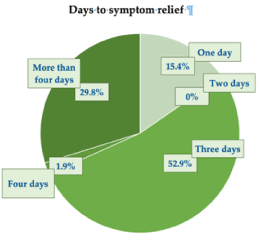
Figure 5.Time (days) elapsed before the cohort subjects reported symptom relief
Safety
Treatment with the PNs/HA-based oral medical device was well tolerated, with no reported local or systemic adverse effects, unexpected side events or exposure to unknown risks.
Conclusion
Unlike the somewhat artificial setting of randomized controlled trials, real-world studies provide insights into clinical outcomes and safety in conditions much like everyday clinical practice. The survey format allows for information collection in less time than personal interviews [16].
Real-world studies are most useful in the oral care of persistent and recurrent ulcers and erosions due to clinical polymorphism and the wide range of underlying conditions that manifest with painful oral lesions. Recurrent aphthae-like lesions also have a high incidence, and their psychological impact is persistently severe. A solid rationale — passively replenishing the tissue pool of nucleosides, nucleotides and other nitrogen precursors without any pharmacological intervention [5] —suggests that PNs may be helpful to counteract the loss of richly innervated oral tissues and the ensuing severe pain, as already proved in many other indications, including the troubling, complex problem of torpid wound healing [6-11]. The demonstrated effect of increased collagen production by gingival fibroblasts gives a rational basis to the accelerated healing of oral ulcers and even lesions due to radiation-induced skin and mucosal toxicity [17,18].
The survey showed that, on average, in a prospective cohort of individuals representative of the general population, the monitored PNs/HA-based device accelerated the healing and physiological recovery of oral lesions, whatever the underlying condition. Moreover, it rapidly and consistently suppressed the main six symptoms associated with oral ulcers and related conditions: pain, irritation and discomfort whatever the triggering event (chewing, swallowing, talking, contact with the tongue, irritating foods). Almost seven subjects out of ten reported symptom relief within three days, often within one day. Suppression of pain and discomfort was most remarkable for generalised oral pain and discomfort and pain discomfort due to chewing and irritating foods but mean final oral pain scores never exceeded 1.4 for all symptoms, much less than the moderate severity threshold. The relief of oral symptoms was associated with perfect tolerability with no report of side effects or unexpected risks from the mucoadhesive gel application.
In conclusion, the real-world monitoring study demonstrated that the PNs/HA-based oral care gel retains its long-standing record of efficient performance and excellent safety.
Conflicts of interest
The authors were formerly R&D consultants to Mastelli S.r.l., Sanremo, Italy (PNs producer). They certify that, at present, they have no conflict of interest with any financial or commercial organization concerning the manuscript’s content.
Acknowledgements
The authors wish to thank their colleagues Rosa Talarico, Salvatore Balsamo, Rossana Romanello, and Virgilio Scarcello, all practising in leading oral care institutions in Sicily, Italy, for the contribution of some patients treated following the study protocol. The authors also wish to thank Dr Mauro Raichi, clinical pharmacologist, for helping with analyses and discussion of outcomes and medical writing.
References
- Siu A, Landon K, Ramos DM (2015) Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg 34: 171-177. [Crossref]
- Thakrar P, Chaudhry SI (2016) Oral ulceration: an overview of diagnosis and management. Prim Dent J 5: 30-33. [Crossref]
- Akintoye SO, Greenberg MS (2014) Recurrent aphthous stomatitis. Dent Clin North Am 2: 281-297. [Crossref]
- Gülseren D, Hapa A, Ersoy-Evans S, Elçin G, Karaduman A. (2017) Is there a role of food additives in recurrent aphthous stomatitis? A prospective study with patch testing Int J Dermatol. 56: 302-306. [Crossref]
- Giani I, Elbetti C, Novelli E, Zanieri DG (2013) Polynucleotides and terpinenol: an effective aid in preventing mesh exposure in pelvic floor surgery. Pelviperineology 32: 77-79.
- Segreto F, Carotti S, Marangi GF, Francesconi M, Scaramuzzino L, et al. (2020) The use of acellular porcine dermis, hyaluronic acid and polynucleotides in the treatment of cutaneous ulcers: single-blind randomised clinical trial. Int Wound J 17: 1702-1708. [Crossref]
- Pilloni A, Rojas MA, Trezza C, Carere M, Filippis AD, et al. (2022) Clinical effects of the adjunctive use of a polynucleotides and hyaluronic acid-based gel in the subgingival re-instrumentation of residual periodontal pockets: a randomized, split-mouth clinical trial. J Periodontol [Crossref]
- De Caridi G, Massara M, Acri I, Zavettieri S, Grande R, et al. (2014) Trophic effects of polynucleotides and hyaluronic acid in the healing of venous ulcers of the lower limbs: a clinical study. Int Wound J. [Crossref]
- 20. Araco A, Araco F (2021) Preliminary prospective and randomised study of highly purified polynucleotide vs placebo in treatment of moderate to severe acne scars. Aesthet Surg J 41: 866-874. [Crossref]
- Palmieri IP, Raichi M (2019) Biorevitalization of postmenopausal labia majora, the polynucleotide/hyaluronic acid option. Obstet Gynecol Rep 3: 1-5.
- Angelucci M, Frascani F, Franceschelli A, Lusi A, Garo ML (2022) Efficacy of intradermal hyaluronic acid plus polynucleotides in vulvovaginal atrophy: a pilot study. Climacteric 25: 490-496. [Crossref]
- Garratt AM, Helgeland J, Gulbrandsen P (2011) Five-point scales outperform 10-point scales in a randomised comparison of item scaling for the Patient Experiences Questionnaire. J Clin Epidemiol 64: 200-207. [Crossref]
- Faul F, Erdfelder E, Buchner A, Lang A-G (2009) Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 41: 1149-1160. [Crossref]
- Statistical software downloaded from https://www.analystsoft.com/en/products/statplus/#statfeatures.
- Mishra P, Singh U, Pandey CM, Mishra P, Pandey G (2019) Application of Student’s t-test, analysis of variance, and covariance. Ann Card Anaesth 22: 407-411. [Crossref]
- Miksad RA, Abernethy AP (2018) Harnessing the power of real‐world evidence (RWE): a checklist to ensure regulatory‐grade data quality. Clin Pharmacol Ther 103: 202-205. [Crossref]
- Colangelo MT, Belletti S, Govoni P, Guizzardi S, Galli C (2021) A biomimetic polynucleotides–hyaluronic acid hydrogel promotes wound healing in a primary gingival fibroblast model. Appl Sci 11: 4405.
- Giudici S, Maggio F, Bertocchi M (2022) Topical natural-origin polynucleotides in radiation-induced skin and mucosal toxicity. Review of the rationale and clinical evidence.





