Abstract
This paper contains a brief review of the literature regarding the structure and properties of acrylic bone cements as well as presents their changes generated in the material during its long-term presence in the body. Bone cements sampled during revision surgeries were investigated due to literature reports highlighting the disadvantages of bone cement applications as well as adverse physicochemical phenomena, with particular emphasis on cement shrinkage
during setting as well as alterations of its properties. A study was performed using scanning electron microscopy and confirmed that apart from changes in the properties of the bone cement itself that occur already after a few years of its presence in the body (increased brittleness), a number of adverse phenomena occur at the metal implant - bone cement interface. Fine metal particles bound to the cement surface, which originated as erosion products resulting from the abrasion of implant surface, were observed, while the nature and location of the damaged areas implies lack of cohesion between cement and an attached implant and, consequently, forming of a friction couple. Also, damage to the implant surface due to a detachment of the mounting cement substructure was found in the investigated components. Apart from the damaged connection at the interface between cement and metal implant, anchoring of alloy particles in the cement layer followed by their separation occurred in the area modified during laser marking.
Keywords
bone cement, polyethylene, metal, prosthesis, surgery
Introduction
Although PMMA-based cements are often classified in the group of bioneutral biopolymers, literature reports indicate that indeed the polymer itself is neutral, but it may become toxic due to additives it contains, such as catalysts, stabilizers, plasticizers, dyes, etc. In surgical cements, a monomer known as methyl methacrylate (MMA) has toxic properties.
PALACOS is a bone cement that belongs to the group of self-polymerizing acrylic masses. It is a two-component composition comprised of separately packaged powder component (polymer) and liquid component (monomer). The
polymer component is methyl polymethacrylate (PMMA). The powder is in the form of spherical or irregular particles with developed surface area and with a diameter ranging from fractions to hundreds of micrometers. Polymerization initiator, e.g. benzoyl peroxide, present in the amount of at least 0.5% (Palacos) is an integral part of the powder component, which may occasionally contain a radiological contrast agent such as barium sulphate or zirconium dioxide (Palacos R), in the amount of up to 15%. The liquid monomer component is usually represented by MMA (monomer - methyl methacrylate). The liquid monomer always contains an inhibitor, usually in the amount of 50 – 75 ppm as well as an accelerator such as tertiary amine, typically N,N-dimethyl-p-toluidine
(DMPT) in the amount of at least 0.6% (Palacos). The use of a mixture comprising powdered polymer and only 30% of an unbound polymer allowed to reduce a major disadvantage, i.e. shrinkage due to polymerization. A change in
monomer density from 0.943 kg/m3 to 1.28 kg/m3 throughout the setting would result in volumetric shrinkage of 35%, which could be reduced to about 5% by the above mentioned solution. Additional shrinkage effect of not more than 0.3% is caused by thermal expansion due to temperature changes from polymerization temperature to body temperature. [Bishop N.E., Ferguson S., Tepic S.: Porosity reduction in bone cement at the cementytem interface. J. Bone Joint Surg., 78-B, 3, 1996, p. 349-356]. Even a few percent changes in the volume are undesirable and considered one of the disadvantages of cement implant applications. They may result in the formation of discontinuities and gaps at cement-tissue interface, in particular cement-implant interface, which involves the risk of
loosening. Cement shrinkage combined with locally unfavorable geometry (e.g. implant component in the form of bilaterally limited pocket) may result in loosening and, consequently, formation of a friction couple.
Long-term property stability is an important aspect of quality and stability of cement implantations. Apart from stability in terms of biotolerance, attention should be paid to strength properties whose change may contribute to altered conditions of implant functioning. Studies involving different types of bone cement clearly indicate significant changes in the mechanical properties reaching 10%, which are observed during a period of 2 years [Balin A., Toborek J.: Właściwości użytkowe cementu chirurgicznego. Biology of Sport, vol. 15, supl. 8, 1998, s. 184-188; Lee A.J.C.: Cement strength-relationship with bonecomparison of different available cement. Revision Arthroplasty. Proc. of a Symposium held at Sheffield Univ. 1979, p. 5-17]. Much research was devoted to cement property changes, which occur over time, and confirms that mechanical properties become significantly altered (Figure1).
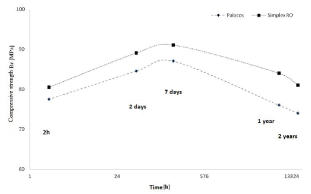
Figure 1. Strength alterations determined in the static compression test, dependent on the “age” of the bone cement [based on: [3]
Such significant property changes observed for Palacos and Simplex cement involve the risk of alterations in the properties of tissue-cement-implant interface, which, in the aspect of the risk involved in the potential implant
disturbances, necessitates bone cement evaluation after its long-term presence in the body. The aim of the thesis was to present the effects of bone cement wear.
Results
The following study presents bone cement evaluations after different periods of use. Fracture morphology of bone cement prepared for the evaluation as reference material ‘1’ (Figure 2a) and bone cement sampled during a revision
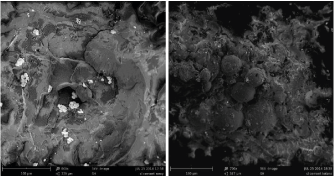
Figure 2. An image of the fracture in the cement prepared 14 days prior to assessment (a) and
obtained during a resurgery, 12 years after implanting (b)
surgery after 12 years ‘2’ (Figure 2b) was assessed in order to verify the risk of adverse phenomena. Structural studies using scanning electron microscopy (accelerating voltage of 5kV was used due to the properties of PMMA polymer) indicate structural differences in the fracture (Figure 2). In both cases, a characteristic structure of the cement in the form of ceramic phase particles distributed in polymer matrix is observed. A number of lateral cracks originating from the main crack as well as changes in the direction of cracking once the fracture has reached ceramic particles are observed on the fracture surface of sample 1. Sample 2 fracture shows a different pattern of cracking. No accompanying cracks were observed in this case, but only the major fracture plane. This may result from an increased material brittleness, and the lack of accompanying cracks indicates that the amount of energy needed to destroy sample 2 was lower compared to sample 1. Furthermore, main fracture plane propagated in such a way that spherical particles of the polymeric phase added as a filler became noticeable. This type of cracking may indicate lower cohesion of the material in the interface area of polymeric particles and the matrix that polymerizes during cement setting. This effect was observed only in the case of materials recovered from the body after 12 years.
Cement surfaces from the side of metal implant were also investigated. Samples were taken from several areas of complete and partial knee replacement as well as from an artificial acetabulum (Figure 3). Portions of study material were sampled from the areas indicating insufficient connection as well as the presence of fissure between the cement layer and the implant layer, which may indicate the site of loosening.
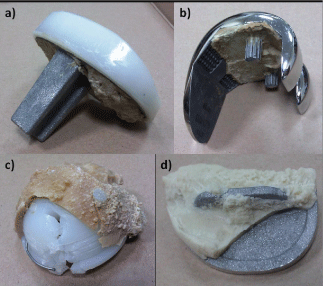
Figure 3. Examples of the investigated implants
In the case of two (Figures 3a and b) out of four investigated implants, apart from ceramic particles, fine metallic particles of implant material were also found. This indicates the occurrence of micromotions during implant use as well as formation of erosion products as a result of abrasion (Figure 4). The external surface was characterized by a complex topography, which was formed as an imprint of sandblasted implant surface. The formation of friction pair at cement – implant interface is further indicated by the presence of small, flat areas comprised of abrasion marks arranged in one direction, which were certainly formed as a result of an interaction with implant surface. Furthermore, implant surface damage caused by long-term abrasive interaction with the bone tissue was observed (Figure 3a). The rough surface of the implant (Figure 5a) at the damaged sites was glossy (Figure 5b), and it should be noted that the abrasive products in the form of fine alloy particles contributed to medical complications and resurgeries. The baseline surface quality with the Ra parameter of 1.8μm decreased to 0.6μm at the sites of interaction with hard tissues.

Figure 4. The surface of bone cement seen from the side of metal implant with noticeable fine
erosion product particles
Further studies also showed damaged implant surface due to contact with bone cement. The investigated portion was sampled from the area where serial number was applied during implant formation (Figure 3d). Bone cement layer was not bound to the implant surface and was mechanically separated from the major portion. Metal particles were separated from the implant and bound to cement to such an extent that part of the serial number in the form of a negative could be seen on the cement surface (Figure 6). An exemplary fragment of implant marking imprinted on the cement surface is shown below (“12”) (Figure 6a). The revealed sign was formed as a result of separation of alloy particles from the area of laser marking, and the surface of metal particles bound to cement indicates a cyclic

Figure 5. Sandblasted implant surface in the intact area (a) and in the damaged area (b)
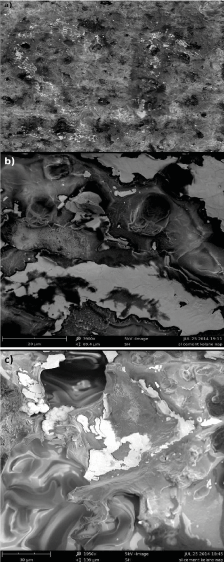
Figure 6. The surface of cement sampled from the site of serial number application, with noticeable alloy portions that separated from the implant
contact resulting in gloss at the site of contact.
Conclusions
Relevant literature analysis and the conducted studies indicate that changes in the properties of the bone cement, which are manifested by a different cracking pattern and which contribute to an increased material brittleness, occur after a period of 12 years. Furthermore, the studies indicate that a number of adverse phenomena take place at implant-bone cement interface. Metal abrasive wear products bound to the bone cement surface as well as implant surface damage at the site of marking were identified. Hip implant surface also shows signs of wear that result from the contact with tissues. The investigated components of an artificial knee joint do not clearly indicate whether implant loosening resulted from the properties of bone cement or if loosening due to other reasons contributed to implant surface damage and adverse phenomena at implant – cement interface. However, it should be noted that the use of cement involves an increased risk of complications that result from altered properties of the cement itself as well as additional separation surfaces being formed (implant – cement, cement – tissue), which represent areas where adverse abrasive wear processes may take place.
JS designed the study, performed revisions of hip and knee replacements, wrote the article and collected data. SL performed the data analysis. PG corrected the article and assisted in interpreting the results. DK assisted in interpreting the results.
The work presented here was a collaboration between all the authors, who contributed to and approved the manuscript.
The study was performed at the Department of Orthopaedic Surgery in Luxmed Hospital, Warsaw, Poland and at the Department of Advanced Materials and Technology, Military University of Technology, Warsaw, Poland. All activities were funded by JS.
No competing interests declared.
The article was written on the basis of 30 years of experience and practical knowledge acquired during performing of arthroplasty (mainly arthroplasty of hip and arthroplasty of knee) and research on biologically active substances in the treatment of motor organs in cooperation with the Military Technical Academy and the Biochemistry Unit of the Medical University in Toruń. The need to undertake this type of research was determined by the large number
of revision operations after prosthesoplasty surgeries and the rapidly progressing loss of bone mass in the vicinity of loosened implants.
References
- Bishop NE, Ferguson S, Tepic S (1996) Porosity reduction in bone cement atthe cementytem interface. J Bone Joint Surg 349-356. [Crossref]
- Balin A, Toborek J (1998)Właściwościużytkowecementuchirurgicznego. Biology of Sport 15: 184-188.
- Lee AJC (1979) Cement strength-relationship with bone-comparison ofdifferent available cement. Revision Arthroplasty. Proc of a Symposium heldat Sheffield Univ 5-17.






