Amiodarone is an antiarrhythmic drug commonly used in treatment of arrhythmias. It is considered a safe drug both used intravenously in life-threatening condition and orally in chronic administration. Acute liver failure is a rarely reported adverse effect of intravenous administration of amiodarone. We described a case report of a 76-year-old Caucasian male with persistent atrial fibrillation who developed acute liver failure shortly after intravenous administration of 300 milligrams of amiodarone due to increased ventricular rate. The laboratory results showed: ALT 4376 U/L, AST 5139 U/L, total bilirubin 1.91 mg/dL, INR 2.1, ammonia 85 µg/L, alkaline phosphatase 55 U/L, gamma-glutamyl transpeptidase 101 U/L. Viral hepatitis, ischemia and influence of other hepatotoxic drugs were excluded. Despite numerous comorbidities (ischemic heart disease, chronic heart failure, history of ischemic stroke, acute kidney injury and diabetes type 2), a successful course of the treatment was observed, and liver function normalized within 3 weeks.
acute liver failure, adverse drug reaction, amiodarone, hepatotoxicity, polysorbate 80
Amiodarone is an antiarrhythmic drug widely used to treat atrial fibrillation and ventricular arrhythmias. Due to the long half-life, lipophilic structure and accumulation in the thyroid, liver, lungs, cornea and skin, this drug has many side effects [1]. Acute liver failure (ALF) is a rare complication following the administration of amiodarone [2].
A 76-year-old Caucasian male with type 2 diabetes, persistent atrial fibrillation, after ischemic stroke and myocardial infarction, treated with rivaroxaban, metformin and insulin, was admitted because of palpitation with irregular heart rhythm 120-150/min and BP 110/60 mmHg. The transaminases and troponin levels were in normal range, although urea (122 mg/dL) and creatinine (1.7 mg/dL) levels were increased. In the ultrasound of the abdomen cholecystolithiasis was diagnosed, without a suspicion of cholecystitis. In ECG: atrial fibrillation, HR~150/min. Because of unsatisfied heart rate control with beta-blocker, 300 mg of amiodarone was administrated intravenously in 60 minutes. Laboratory tests 12 hours after amiodarone administration revealed critical increased AST activity (Figure 1). Patient was in stable condition, abdomen was without pathological signs, however he was somnolent, and then disorientated, what was considered to be a sign of encephalopathy. The next laboratory results showed: ALT 4376 U/L, AST 5139 U/L, total bilirubin 1.91 mg/dL, INR 2.1, ammonia 85 µg/L, alkaline phosphatase 55 U/L, gamma-glutamyl transpeptidase 101 U/L. Ultrasound scan and computed tomography didn't reveal any other changes in the liver. Viral hepatitis, ischemia and influence of other hepatotoxic drugs were excluded. Echocardiography revealed enlarged atria and left ventricle, akinesis of the anterior wall and septum, EF 30% as well moderate mitral and tricuspid regurgitation. Intravenous fluids, metoprolol, digoxin, insulin, furosemide and vitamin K were used, the diet was gradually expanded, and improvement of the clinical condition and the results of laboratory tests were observed. After 24 days normalization of all liver parameters was achieved, and patient was discharged home.
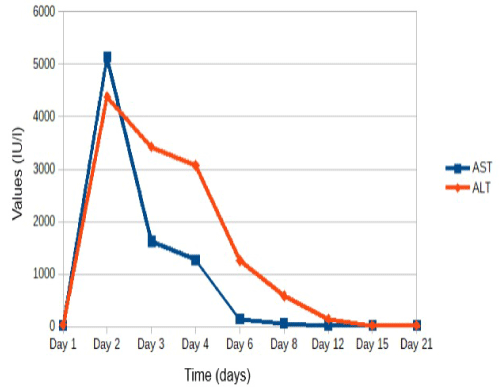
Figure 1. Aspartate (AST) and alanine transaminases (ALT) serum activity during hospitalization
First case report of ALF following amiodarone was described in 1986 [3]. Since then only a few cases have been reported, with high mortality rates [2,4]. Frequency of AFL after amiodarone is estimated at <1% [2]. Diagnosis is made, when following intravenous or oral administration of amiodarone occurs AFL with encephalopathy and coagulopathy. Mechanism of this process is unknown. It is different for intravenous and oral amiodarone administration. Orally amiodarone can cause in a few months a moderated increase of aminotransferases activity but also ALF [2]. It is necessary to exclude ischemic hepatitis, in which liver failure is a reaction to hypoperfusion [5]. In the reported case imaging studies excluded abnormalities in the liver and main abdominal vessels. The symptoms are varied but usually the course is life threatening, it can be progressive, irreversible but also reversible [2,4]. Treatment is difficult, because guidelines for managing this condition have not been published yet. It is mandatory to discontinue amiodarone and to provide care in intensive care unit using intravenous fluids, pressure amines, and adequate enteral or parenteral nutrition. Such treatment, as in described case, proved to be successful [2] but more often there are cases in which the treatment has resulted in death [2,4].
- Grabczak EM, Zielonka TM, Wiwała J, Bareła AD, Opuchlik A, et al. (2008) Amiodarone induced pneumonitis and hyperthyroidism: case report. Pol Arch Med Wewn 118: 524-529. [Crossref]
- Jaiswal P, Attar BM, Yap JE, Devani K, Jaiswal R, et al. Acute liver failure with amiodarone infusion: A case report and systematic review. J Clin Pharm Ther 2018; 43: 129-133. [Crossref]
- Lupon-Rosés J, Simó-Canonge R, Lu-Cortez L, Permanyer-Miralda G, Allende-Monclús H (1986) Probable early acute hepatitis with parenteral amiodarone. Clin Cardiol 9: 223-225. [Crossref]
- Grecian R, Ainslie M (2012) Acute hepatic failure following intravenous amiodarone. BMJ Case Rep: bcr2012007080. [Crossref]
- Gluck N, Fried M, Porat R (2011) Acute amiodarone liver toxicity likely due to ischemic hepatitis. Isr Med Assoc J 13: 748-752. [Crossref]

