A recent information processing model of two-choice RT situations, suggests that conditions which reduce the duration of peripheral motor processes, should also reduce the efficiency of the action monitoring system, because letting no time enough for correction of partial errors (i.e. subthreshold transient muscle activity of the agonists of the incorrect response preceding the correct response). A physiological situation, namely sustained physical exercise, has repeatedly been reported to reduce the duration of response execution. Therefore, in order to test the prediction of the model, we compared action monitoring efficiency between a sustained exercise (59.42% of MAP) and a control (15 W) condition in the same subjects while they were performing a Simon task. Electromyographic (EMG) recordings of muscles implicated in the response allowed to measure premotor time (time interval between the stimulus and the onset of the EMG burst) and motor time (MT, time interval between the onset of the EMG burst and the mechanical response, which gives access to response execution processes). Electromyogram further permitted to unmask partial errors. Correction ratio was calculated by dividing the number of partial errors by the number of incorrect activations (partial errors + errors). As expected, exercise decreased MT. In addition, exercise reduced the correction ratio. Furthermore, there was a positive inter-subject correlation between these two dependent variables. In line with Servant et al.'s model [1], we propose that the drop in the efficiency of cognitive control was due to insufficient MT available for action monitoring to operate when incorrect activations were produced.
motor time, correction ratio, action monitoring, simon task, physical exercise
Reducing the duration of motor execution processes also reduces the time available to detect and correct an incorrect activation. Therefore, we provide evidence that performance can be impaired, not only by decreasing the efficiency of the information processing chain or by hampering action monitoring operations, but also by reducing the time available for these operations to occur.
It is often assumed that performance, when realized under time pressure, is controlled by a supervisory system that monitors ongoing actions [2,3].
In between-hand choice-RT-tasks or in Go/Nogo tasks, small subthreshold EMG activations of incorrect response agonists, preceding the correct response, are assumed to reflect the detection, inhibition and correction of erroneous responses. For this reason, these subthreshold incorrect EMG activations have been called “partial errors” [4-8]. The existence of partial errors in RT tasks is among the experimental pieces of evidence in favor of the existence of a supervisory (or action monitoring) system acting “online” [9,10].
Partial errors allow evaluating the efficiency of the supervisory system. Indeed, the ratio between the number of partial errors and the number of incorrect activations (i.e. partial errors + full-blown errors), called “correction ratio” represents the ability to suppress covert incorrect activations to prevent overt errors [11]. Lower correction ratios reflect less efficient error suppression and, as a consequence, reflect less efficient action monitoring processes.
Now, Servant et al.’s [1] recent model suggests that the duration of response execution as measured by the motor time (MT) can affect incorrect activation suppression (i.e., the correction ratio) because incorrect activations suppressions are implemented until the very end of the MT. The authors enriched previous diffusion models of decision-making [12,13] and relied on the idea that the accumulation of evidence continues during motor activity, even down to the effectors contraction [1]. Instead of one classical threshold or bound needful to trigger motor activity, they described two types of bound for the response selection process: i) internal EMG bounds that trigger EMG activation and ii) standard bounds that determinate the decision and trigger a mechanical response. After an EMG bound is hit, evidence continues to accumulate until a decision determination bound is reached, giving the models potentiality to overcome incorrect EMG activation thanks to time-varying drift rate dynamics. The time interval between the onset of the EMG burst (i.e., the first bound is reached) and the mechanical response (i.e., short time after the second bound is reached) corresponds to the MT. Incorrect activation suppression takes time to build up. The longer the MT, the more chances this process can be implemented and the more chances an incorrect activation can be corrected before it turns into an overt error. In support of this notion, the authors reported that, as predicted by their model, there was an inter-subject correlation between partial error rates and MTs: the shorter the MT of correct responses, the smaller the partial error rate. Now, one can derive from this model that the reduction of partial error rate with MTs should be due (at least in part) to a reduction of the correction ratio for shorter MTs. In other words, the ability to suppress incorrect activation should be inversely linked to the duration of MT. As a consequence, this model suggests that the efficiency of the action monitoring system can be reduced because of peripheral inadequate conditions, while the action monitoring system in itself is not impaired.
Physical exercise is a physiological situation in which the MT is quite systematically reduced. Indeed, in different types of RT tasks, it has been demonstrated that the reduction of RTs observed at exercise is largely due to a reduction of MT; This was the case in a simple RT task [14], but also in choice RT tasks [15,16]. Recently, Beyer et al. [17] did observe a reduction of RTs at exercise that was exclusively due to a reduction of MTs. According to Davranche et al. [15], the effect of exercise on MT exercise could be linked to the recruitment of the sympathoadrenal system by the hypothalamus that increases the synthesis and release of norepinephrine and dopamine in the brain [18]. Therefore, if exercise shortens MTs, as it seems to be the case, one can expect, according to Servant et al. [1] model, that exercise, should also reduce the correction ratio by diminishing the time available for incorrect activations suppression to occur.
Schmit et al. [19] used a conflict RT task in which three categories of trials were distinguished thanks to responses agonists EMG recordings: (i) pure-correct trials, i.e., correct trials containing no partial error; (ii) partial error trials, i.e., correct trials containing a partial error; and (iii) error trials in which supra-threshold incorrect activation resulted in a full-blown error. The authors observed that the correction ratio was reduced just before exhaustion and concluded to a decrease in action monitoring processes efficiency since this reduction of the correction ratio was associated to an increase of the error rate. Now, the authors, using Near Infrared Spectroscopy of the right inferior frontal cortex (rIFC) to explore a possible dysfunction of a structure supposed to subserve executive functions (to which action monitoring belongs), could not evidence any oxygenation drop below baseline values.
Using other indices of action monitoring, Davranche et al. [20] did not obtained either any evidence of action monitoring dysfunctions at exercise. These authors resorted to a Simon task [21] that provides experimental contexts and theoretical models for analyzing how irrelevant external stimulus information elicits response impulses that interfere (i.e., conflict) with goal-directed actions and how these impulses can be actively suppressed [21-23]. Davranche et al. [20] sought to evaluate possible post-error adjustments impairments and/or possible impairments of inhibitory control at different levels of exercise (light, moderate, and high intensity). The authors obtained no evidence for reduction of impulse suppression at exercise nor did they observe any reduction of post-error adjustments. On the contrary, they evidenced a RT reduction for all exercise conditions while the error rate was maintained constant. Considering that exercise quite systematically reduces MTs, it is to be noted that this RT reduction was likely due, at least in part, to a reduction of MTs.
To sum up, exercise has been reported to reduce MTs [14-17] and correction ratio [19] but does not seem to affect significantly action monitoring processes [19,20]. Therefore, exercise seems to represent a very convenient physiological condition to put to the test the predictions of Servant et al.’s [1] model.
In the present study, we submitted subjects to a task very similar to that of Davranche et al. [20]. Subjects had to perform a Simon task either in a control condition or during a sustained exercise, long enough (20 minutes) to obtain a substantial number of trials, while their RTs, error rates and response agonist EMG activities were measured. We predicted: (1) a shortening of RTs [20] which would be partially or completely explained by a reduction of MTs [14-17] ;(2) a reduction of the correction ratio [19] due to insufficient time let for correction of incorrect activations [1]; and (3) a positive inter-subject correlation between MTs and correction ratios.
Participants
Sixteen healthy volunteers volunteered for the experiment. Participants were nurse students, medical students or personals from the Institute and they were recruited either from the military nurse school or from the Val de Grace military school. Table 1 summarized their anthropometrical features. This study was approved by the Marseille II ethic comity. All subjects signed a written informed consent prior to their participation.
Table 1. Participants’ characteristics (p values were obtained with student-tests to evaluate differences between males and females)
Variables |
All |
Male |
Female |
p |
Sample size |
16 |
11 |
5 |
|
Age (years) |
30.6±8.6 |
30.6±9.6 |
30.6±6.9 |
0.99 |
Body mass index |
22±2 |
22.4±2.2 |
21.1±1.8 |
0.26 |
VO2max (ml/kg/min) |
47.3±7.8 |
51.0±6.5 |
40.0±4.2 |
0.005 |
Maximal power (W) |
269±63 |
290±59 |
220±43 |
0.03 |
Cognitive task
The present study relied on a common version of the Simon task in which the subjects have to choose between a left- and a right-hand key press according to the colour of a visual stimulus presented a few degrees either to the left or the right of a visual fixation point. Performance expressed both in terms of error rate and RT is better when the required response corresponds spatially to the irrelevant stimulus location (congruent association) than when it does not correspond (incongruent association). This effect is termed the “Simon effect” [24,25]. A widely accepted interpretation of the Simon effect is that the irrelevant stimulus location automatically engages a response impulse ipsilateral to the stimulus while the relevant stimulus colour must be translated into the required response according to the task instructions [26-28]. When the stimulus-response association is congruent, the impulse triggered by the irrelevant stimulus location activates the required response, which facilitates response processing. In contrast, when the stimulus-response association is incongruent, the impulse triggered by the irrelevant location activates the non-required response which must be suppressed and replaced by the required one. These additional operations occur at a cost and the performance is degraded.
Previous research showed that incorrect activations (full performance errors and partial errors) occur for both congruent and incongruent associations, but more frequently for incongruent ones [29]. The difference in the frequency of incorrect activations between incongruent and congruent associations reflects the expression of the ipsilateral impulses triggered by the irrelevant stimulus location [8].
Apparatus and stimuli
Participants faced a computer screen on which the stimuli were presented. The distance between the screen and the subject’s eyes was 1.40 m. A black cross (7 mms height, 2 mm width) displayed on the centre of the screen served as a fixation point. The stimuli were blue, yellow, red and green filled circles (40 mm in diameter) delivered either to the left or to the right of the fixation point. The distance between the two possible stimulus locations subtended 6° of visual angle. The response was a left or right index finger press of at least 7.8 N on laterally positioned force sensors (Mescan LK-SS 50).
The task was performed on a cycle ergometer (Corival®, Lode B.V., Groningen, the Netherlands) in a room where ambient temperature and relative humidity were controlled not to exceed 22°C and 60%, respectively. The handlebar was fitted with a shelf to support the arms of the participants. The two response sensors were fixed on the shelf (15 cm between the sensors).
A trial began with the presentation of colored circle either to the right or to the left of the fixation point. Participants were required to respond to the stimulus by pressing either the right or the left sensor, as fast and as accurately as possible. Half the subjects had to produce a right response to the presentation of a red or green circle, or a left response to the presentation of a yellow or a blue circle; this mapping was reversed for the other half. After the response, the stimulus disappeared and the next stimulus was displayed 500 ms later. If no response was given within 800 ms, the stimulus was turned off and the next one was displayed 500 ms later.
A whole set consisted of 10 blocks of 129 trials. A block lasted about 2 minutes and 30 seconds break was given between two blocks. Congruent and incongruent trials were equally distributed within each block.
Data recordings
The electromyographic activity (EMG) was recorded by two Ag-AgCl electrodes fixed about 20 mm apart from one another on the skin over the first dorsal interosseus because this muscle is the prime mover in index finger flexions performed in reaction times tasks [29]. The EMG activity was amplified (gain 5000), the sampling rate was 2048 Hz and bandwidth: DC-400 Hz (3dB/octave). Variations of the developed force were recorded through force sensors (Mescan LK-SS 50) placed under each response key.
Experimental procedure
Three sessions were scheduled and conducted in the morning hours (from 9:00 am to 12:00 am). For each session, subjects were requested to refrain from any strenuous exercise the day before the session, not to experience sleep deprivation and to avoid caffeinated beverages the morning of the session.
During the first session, anthropological features were recorded and a familiarization with the cognitive task was performed. Subjects completed at least 3 blocks of 100 trials of the Simon task. Then a determination of maximal oxygen uptake (VO2max) was undertaken for all subjects on the cycle ergometer. Power output was automatically adjusted in order ensure a constant power whatever the pedal frequency. An incremental protocol was used: after a 4 minutes warm up at light intensity (men: 80 W; women: 70 W) the workload was increased of 10W every 30 seconds until exhaustion. Participants were encouraged to achieve their maximal level. Exhaustion was defined when subjects could no longer maintain a pedaling frequency above 50 rotations per minute in spite of vigorous verbal encouragement. Oxygen consumption and ventilatory output were recorded using the Fitmate Pro gas analyzer (COSMED®, Miami, FL, USA). Heart rate was recorded by a cardiac belt (Polar RS800CX, Polar Electror Oy, Kempele, Finland).
The second and third sessions were experimental sessions. They consisted in an exercise condition and a control condition. The order of the condition was randomly assigned between the participants and balanced every 4 subjects. In the control condition, subjects were required to pedal at 70 rounds per minute with a pedal resistance set to 15 W. In the exercise condition, the intensity was set at the power corresponding to the first ventilatory threshold with an extra load of 5%. Albeit10 subjects maintained this exercise intensity during 25 minutes (61.43% of MAP), 6 subjects were unable to maintain this intensity for the duration of the exercise session. The exercise intensity was therefore adjusted; their average pedaling power was 89% of the power reached on the first ventilatory threshold (56.06% of MAP). On average, the pedaling power of the 16 subjects was 59.42% of MAP (min46%-max 79%; standard deviation 8%) (Figure 1).
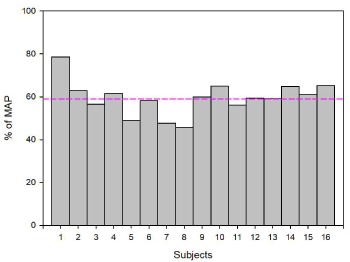
Figure 1. Percentage of Maximum Aerobic Power (MAP) developed on exercise session for each subject
Data analyses
EMG signals and force levels were analysed with Brainvision Analyser 2.1.
EMG signal analysis: On EMG activities, we performed continuous wavelet transformation, using Morlet Complex. Spectral amplitude (real values in µV) were measured from 10 to 400Hz, 7 as Morlet parameter, frequency steps of 100 and using logarithmic steps. These analyses were performed during the EMG activities of correct and partial error trials, in both the activated and non-activated response agonists. The analysis time-window extended from EMG onset to 80 ms post-EMG onset (corresponding to about the first 80% of MT of correct trials). The impact of effort condition (exercise or control) on EMG activity was investigated through the analysis of the HF/LF ratio (HF: high frequencies, LF: Low frequencies). Low frequencies were represented by a10 to 110Hz frequency interval and high frequencies by a300-400 Hz frequency interval. These measures were submitted to an analysis of variance involving factors condition (control or exercise) and congruency (congruent or incongruent).
On the one hand, the analysis of the HF/LF ratio of the muscle implicated in the production of the response aimed at exploring the temporal recruitment in the control and exercise conditions. Indeed, for distal muscles as the first dorsal interosseus of the hand, the firing rate plays a prominent role in force modulation [30]. Furthermore, Christensen et al. [31], described that at moderate to high force the HF/LF amplitude ratio increased. On the other hand, the analysis of the HF/LF ratio of thenon-activated muscles aimed at evaluating the global muscle tone in the exercise and control conditions.
Moreover, EMG traces were visually inspected and EMG onsets were hand-scored [32] by an experimenter blind to the stimulus-response association (congruent versus incongruent) and the type of the effort during the session (exercise versus control). Trials were classified as (i) pure-correct: a mechanical response on the required sensor, (ii) error: a mechanical response on the non-required sensor, and (iii) correct trial containing a partial error (Figure 2). To be classified as a partial error trial, the EMG signal deflection had to be phasic and return to baseline (rest) level before the onset of the EMG activity related to the button-press response. Pure-correct responses, errors and partial errors were detected and counted. RT was defined as the delay separating the presentation of the stimulus and the mechanical response (correct or error). RT was fractioned (Figure 2) into premotor time (PMT) and motor time (MT). The PMT was defined as the delay separating the presentation of the stimulus andthe onset of the EMG burst. The MT was defined as the delay separating the onset of the EMG burst and the mechanical response [2]. For correct trials containing a partial error, partial error latency was defined as the delay separating the presentation of the stimulus from the onset of the EMG burst of the partial error.
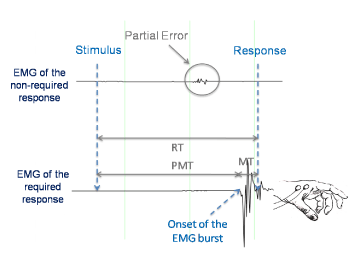
Figure 2. Electromyographic (EMG) activity for a correct response containing a partial error. The correct response was elicited on the required side after a subthreshold EMG burst was recorded on the non-required side. MT: motor time; PEL: partial error latency; PMT: premotor time; RT: reaction time
RT, PMT ant MT were submitted to analyses of variance involving two within-participants factors: condition (control, exercise) and congruency (congruent, incongruent).
In addition to usual analyses of the mean values of chronometric data, we performed distribution analyses of MT. To this aim, we used the “Vincent averaging” or “Vincentization” technique [33,34]. To make these analyses, we binned the MT distribution of pure-correct responses in seven classes of equal size (same number of trials) and we computed the mean of each class. MT were analyzed by means of an analysis of variance involving three within-participants factors: Effort condition (control, exercise), congruency (congruent, incongruent) and Bin.
Error rate was defined as the number of overt errors, divided by the number of all trials, i.e. pure-correct, correct containing a partial error and errors. Incorrect activations were the summation of partial errors and overt errors. An incorrect activation occurred each time an EMG burst appeared on the side opposite to the required response; when it could be corrected before it reached the mechanical threshold of the mechanical response, it produced a partial error; when it reached the mechanical threshold of the mechanical response, it produced an error. Incorrect activation rate was then defined as the number of trials that elicited partial or full-blown errors divided by the total number of trials.
Correction rate was defined as the number of partial errors (i.e., incorrect activations that could be corrected on time) divided by the number of incorrect activations (partial errors + errors).
Error location function and error location index
We assessed the effect of physical exercise on the strength of the automatic response using a new measure of the strength of the automatic response recently proposed by Servant et al. [35]. The Error Location Function (ELF) represents the proportion of errors located below each quantile of the overall RT distribution. Here, ELF represented the proportion of incorrect activations below each quantile of the overall PMT distribution and was computed on the control condition and on the exercise condition. Specifically, we quantified the strength of the automatic response by the Error Location Index (ELI), which is equal to the area under the ELF curve. ELI can be interpreted as the expectation that a uniformly drawn incorrect response is faster than a uniformly drawn (correct or incorrect) trial. Thus, if ELI = 1, all incorrect activations are concentrated among the fastest trials, which corresponds to a very strong response capture. On the other hand, if ELI = 0, all incorrect activations are concentrated among the slowest trials, which is the converse of response capture. In general, a higher ELI indicates a stronger response capture. An analysis of variance was performed on ELI values to assess the effect of congruency and of exercise on the automatic response strength with factors congruency (congruent or incongruent) and condition (exercise and control).
Force level analysis
To explore the force developed on control and exercise conditions, we averaged the level of force time-locked on the maximum of amplitude of force (zero of time). Level of force was evaluated by (i) rising slope of force in the time window [-100, 0] ms, to evaluate the speed of force increase for pure-correct trials and in the time window [-80, 0] ms for partial errors, and (ii) amplitude at zero of time, corresponding to the maximum force developed by subjects. These measures were submitted to an analysis of variance with factors condition (control or exercise) and congruency (congruent or incongruent).
Pure-Correct trials
As shown on Table 1, RT, MT and PMT were shorter on congruent trials than on incongruent trials (F (1,15) = 59.14, p<0.001;F(1,15)= 14.91, p< 0.01 and F(1,15)= 51.77, p< 0.001, respectively) whatever the condition (control or exercise). Exercise was associated with shorter RTs(F(1,15)= 10.81, p < 0.01) and MTs (F(1,15)= 25.35, p< 0.001) but not significantly shorter PMTs (F(1,15)= 2.97, p=0.105), whatever the congruency of the stimulus-response association. There was no interaction between congruency and condition whatever the studied time interval (RT: F(1,15)= 1.24, p=0.28, PMT: F(1,15)= 1.07, p=0.31 or MT: F<1).
MT distribution of pure-correct trials
MT distributions (Figure 3) were submitted to an analysis of variance with factors condition (control and exercise), congruency (congruent, incongruent) and bin (7 bins) as within-subjects factors. The main effect of condition was confirmed. Condition and bin interacted (F(6,90) = 7.23; p<0.001) and this interaction showed that the effect of condition for each bin was significant. In contrast, both effects were homogeneously distributed throughout the MT distribution.
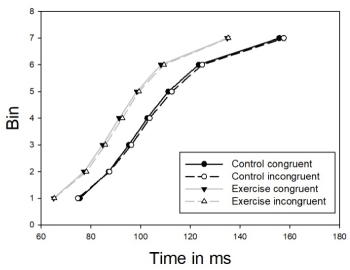
Figure 3. Motor Time Cumulative Density Functions. Congruent stimulus-response association (solid), incongruent stimulus-response association (dashed), control condition (black) and exercise condition (gray)
Level of force for correct trials
Level of force for correct trials (Figure 3) was evaluated with slopes and amplitudes. The analysis of variance revealed a main effect of exercise (F(1,15)= 28.06, p<0,001).As one could expect, according to the shape of curves, slopes were not statistically sensitive to congruency (F<1). The interaction between congruency and effort was far from significance level (F<1).
If one admits that slopes, in the same time window, index the speed of force development, subjects developed their force faster on exercise condition than on control condition.
Amplitude was measured at the zero of time, corresponding to the maximum of amplitude. As for slopes, analysis of variance revealed a main effect of effort condition (F(1,15)= 9.16, p<0,01): force amplitude being larger on exercise than on control condition. It did not reveal any congruency effect (F<1) and there was no interaction between the two factors on this dependent variable (F<1).
To sum up; at exercise, the maximum level of force was reached faster and was larger compared to control condition.
Note that these results are coherent with those obtained on MT. MT was shorter on exercise condition than on control condition because the delay to reach the response criterion (7.8 N) was shorter.
Level of force for partial errors
As for correct trials, force level was analyzed for partial errors (Figure 4). Zero of time corresponded to the maximum of amplitude of force of partial errors. Slopes were estimated from -80ms before the maximum of amplitude to the maximum of amplitude (zero of time). There was a main effect of effort (F(1,15)= 9.99, p<0,01). Slopes were not sensitive to congruency (F(1,15)= 3.74, p=0.07) but a trend was observed in favor of incongruent trials compared to congruent trials. The interaction between congruency and effort was not significant (F(1,15)= 1.46, p=0.25).
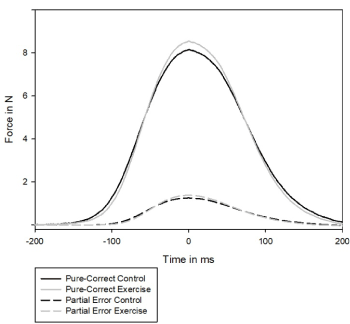
Figure 4. Averaged level of force time-locked to the maximum of amplitude (zero of time) for correct trials, in black on control session and in grey, on exercise condition
Amplitude was measured at the zero of time, corresponding to the maximum of amplitude. As for slopes, an analysis of variance on amplitude revealed a trend for a congruency effect (F(1,15)= 3.44, p=0.08) with higher amplitude on congruent stimulus-response association than on incongruent stimulus-response association. In contrast to correct trials, there was no effort effect (F(1,15)= 2.94, p=0.11). No interaction was found (F<1).
In sum, on partial errors, subjects reached the response threshold earlier at exercise than in the control condition. The following analysis conducted on EMG data suggests that this effect is due an increase of muscle tone at exercise as compared to control.
EMG activities
The analysis of variance performed on the HF/LF ratio (HF=high frequencies between 300 and 400 Hz; LF=low frequencies between 10 and 110 Hz) of pure correct trials' activity (Figure 5) of muscle implicated in the response revealed a main effect of effort condition with a higher ratio on exercise condition as compared to control condition (F(1,15)= 6.15, p<0.05).There was no congruency effect (F<1) and an interaction between effort and congruency (F(1,15)= 5.17, p<0.05). This interaction revealed no effect of congruency neither on control (p=0.75) nor on exercise condition (p=0.16) but an effect of effort condition on congruent (p<0.001) and on incongruent trials (p< 0.001) (Post Hoc test, Tukey HSD test)
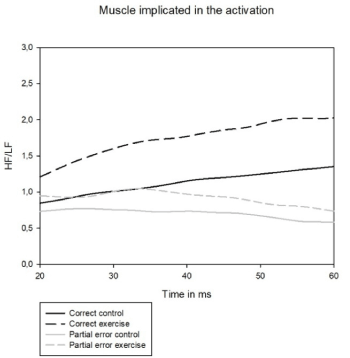
Figure 5. HF/LF ratio for the muscle implicated in the activation, in black: correct activation; in gray: partial error activation; in solid: control condition; in dashed: exercise condition
The increase of HF/LF ratio on exercise condition likely reveals a higher firing rate of motor units at exercise during response execution.
An analysis of variance performed on HF/LF ratio partial errors activity (Figure 5) of muscle implicated in the incorrect activation revealed a mean effect of effort condition with a higher ratio on exercise condition as compared to control condition (F(1,15)= 11.85, p<0.01).Neither congruency effect (F(1,15)= 3.02, p=0.10) nor an interaction between effort and congruency (F<1) were found.
As for correct trials, the increase of HF/LF ratio on exercise condition likely reveals higher firing rate of motor units during partial errors.
The analysis of variance performed on the HF/LF ratio of pure-correct trials' activity (Figure 6) of muscle not implicated in the response revealed a main effect of effort condition with a higher ratio on exercise condition compared to control condition (F(1,15)= 7.69, p<0.05), a congruency effect (F(1,15)= 5.17, p<0.05) and no interaction between effort and congruency (F(1,15)= 2.02, p=0.17).
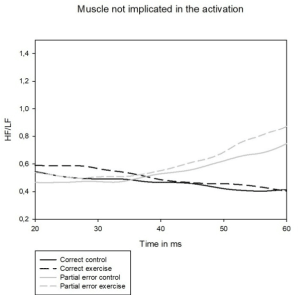
Figure 6. HF/LF ratio for the muscle not implicated in the activation, in black: in the same time as correct activation; in gray: in the same time as partial error activation; in solid: control condition; in dash: exercise condition
The increase of HF/LF ratio on exercise condition likely reveals higher firing rate of the muscles not implicated in the response which suggests a higher muscle tone during exercise as compared to the control condition.
The analysis of variance performed on HF/LF ratio of partial errors activity (Figure 6) of muscle not implicated in the incorrect activation revealed a mean effect of effort condition with a higher ratio on exercise condition compared to control condition (F(1,15)= 5.15, p<0.05). There was no congruency effect (F(1,15)= 2.47, p=0.0.14) and no interaction between effort and congruency (F<1).
As for correct trials, the increase of HF/LF ratio on exercise condition can reflect an increase of muscle tone during exercise compared to control during partial errors.
To sum up, the increase of the HF/LF ratio in the muscle implicated in activation could account for a better motor units recruitment. The increase of the HF/LF ratio in the muscle not implicated in activation during exercise could account for an increase of the global muscle tone during exercise as compared to control condition. Note that the muscles explored in our study are not directly involved in the exercise since subjects had to pedal.
Incorrect activation rate, correction ratio, error rate and partial error rate.
An analysis of variance (Table 2) with factors condition (control and exercise) and congruency (congruent and incongruent) indicated that:
Table 2. RT: reaction time; PMT: premotor time; MT: motor time.CO: Congruent and IN: Incongruent stimulus-response associations ; C: Congruency effect (congruent or incongruent) and E: Effort condition effect (control or exercise).
|
Control |
Exercise |
|
|
CO |
IN |
CO |
IN |
|
RT |
414,43 |
425,70 |
388,61 |
402,25 |
C : p<0.001; E:p<0.001 |
PMT |
307,17 |
317,52 |
294,41 |
307,14 |
C : p<0.001; E:p=0.105 |
MT |
107,26 |
108,18 |
94,20 |
95,11 |
C : p<0.01; E:p<0.001 |
|
|
|
|
|
|
PE rate |
20,03% |
22,30% |
17,46% |
20,54% |
C : p<0.001; E:p=0.06 |
Error rate |
8,76% |
9,66% |
10,39% |
11,61% |
C : p<0.005; E:p=0.09 |
IA rate |
28,79% |
31,96% |
27,85% |
32,15% |
C : p<0.005; E:p=0.70 |
Correction rate |
69,02% |
68,88% |
61,17% |
62,61% |
C : p=0.45; E:p<0.01 |
1) As expected, there was an increase of full-blown and partial error rates on incongruent as compared to congruent condition (error rates (F(1,15)= 5.14, p<0.05;partial error rates F(1,15)= 28.47, p<0.001). There was a trend toward increased error rates at exercise as compared to control condition (F(1,15)= 4.12, p=0.06)and a trend toward decreased partial error rates at exercise as compared to control condition (F(1,15)= 3.38, p=0.09). Congruency and condition neither interacted on error rate (F<1) nor on partial error rate (F(1,15)= 1.11, p=0.31).
2) Regarding the incorrect activation rate, there was an expected increase on incongruent as compared to congruent condition (F(1,15)= 17.09, p < 0.001) with no effect of exercise (F<1) and no interaction (F<1).
3) Conversely, regarding the correction ratio, there was a clear decrease at exercise as compared to control condition (F(1,15) =8.67; p<0.05)with no effect of congruency (F<1),and no interaction (F<1).
To sum up, at exercise, the reduction of the correction ratio (61.89%: exercise vs 68.95%: control), at constant incorrect activation rate (30.00% exercise vs 30.37% control) manifests a pure trade-off between partial and full-blown errors. This trade-off is otherwise reflected in two trends of opposite directions (which resume in the correction ratio): The trends toward increased full-blown error rate and decreased partial error rate at exercise as compared to control condition.
Error location index and error location function
The analysis of variance realized on ELI revealed a main effect of congruency (F(1,15)= 23.49, p<0.001) with an increase of the ELI index on incongruent trials as compared to congruent trials. We found no effect of exercise condition (F<1). The interaction between congruency and exercise was not significant (F(1,15)= 3.51, p=0.08). In other words, incongruent trials exhibited stronger automatic activation than congruent ones and there was no evidence for a stronger automatic activation at exercise as compared to control (Figure 7).
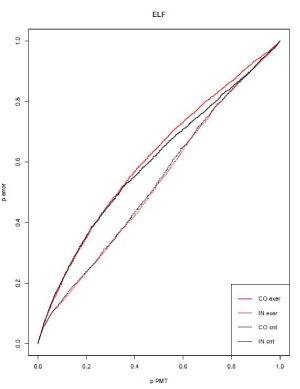
Figure 7. Error Location Function. In fine red: congruent exercise condition, In thick red: incongruent exercise condition, in fine black: congruent control condition and in thick black: incongruent control condition
Correlation between correction ratio and motor time
We averaged the 4 conditions (control congruent, control incongruent, exercise congruent and exercise incongruent) of MT and correction ratio. There was a correlation (r "Pearson" =0.63) between correction ratio and MT (p<0.01). As can be derived from the decision model of Servant and al.(2015), shorter MTs were associated to smaller corrections ratios (Figure 8).
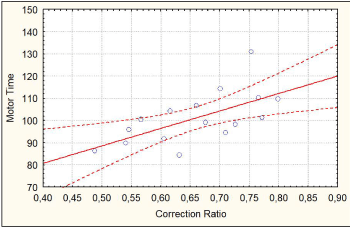
Figure 8. Correlation between MT and correction ratio from Statistica®
To sum up, MTs are decreased at exercise as compared to control condition. Correction ratio is also decreased at exercise as compared to control condition.
Force level analysis (slopes and amplitudes) revealed that subjects press faster and harder the button on exercise condition than on control condition. EMG burst analysis (HF/LF ratio) after continuous wavelets transform) allowed to target a better temporal recruitment of motor units of the muscle implicated in the production of the response and an increase of muscle tone and explains the decrease of MT.
The goal of this study was to determine whether the reduction of MTs, assumed to show up at exercise, would be associated with a reduction of the correction ratio, supporting the idea according to which reduction of MTs would induce a drop in the efficiency of the action monitoring system [1].
Along with Davranche et al., [20], in very similar exercise and task conditions, we observed a reduction of RTs. Along with Davranche et al. and others [14-17], we observed a global shortening of MTs at exercise. Subjects developed a stronger force and reached its maximum level earlier on exercise than on control condition, which resulted in shorter MTs. However, no effect of exercise was reliably evidenced on PMTs, indicating that the reduction of RTs induced by the present exercise was mainly or even exclusively explained by the reduction of the MTs [17]. Given these MTs reductions at exercise, in accordance with Servant et al. [1] model predictions; we observed a reduction of the correction ratio. In accordance with our predictions derived from Servant et al. model, we observed an inter-subject correlation between the MT and the correction ratio.
To sum up, we observed in the same subjects, that MTs and correction ratios were reduced at exercise, these two variables being correlated.
Before discussing the correction ratio it is to be noted that the RTs decrease observed here at exercise does not really reveal a benefit of exercise on information processing processes because this effect can simply be explained by faster response execution due modified muscle tone as will be discussed later; in other words, the effect of exercise performed here is essentially confined to peripheral effects. It is likely that the same effects explain the RTs shortenings previously reported by Davranche et al. [20].
The decrease in the correction ratio observed here at exercise might have been explained by an increase of incorrect activations and more specifically an increase of incorrect activations driven by the automatic route (impulsive activations activated by the location of the stimulus [21]. In this case, such a large increase of incorrect activations would have overwhelmed inhibitory capacities of the action monitoring system, resulting in a decrease of the correction ratio. However, Error Location Index (ELI) and Error Location function (ELF) analyses showed no evidence for a stronger automatic activation by the stimulus irrelevant location at exercise as compared to control condition. Moreover, the incorrect activation rate did not differ between control and exercise conditions. Therefore, ELI, ELF and incorrect activation rates allow excluding an explanation in terms of increased impulsiveness at exercise. Therefore, neither ELI, and ELF nor incorrect activation ratios provide any evidence of impaired inhibitory control at exercise. We will argue in the following that exercise-induced peripheral motor effects can more parsimoniously account for a reduction of the correction ratio at exercise.
Exercise generated a clear peripheral effect which essentially explains the reduction of the RTs in the exercise condition: MTs were reduced at exercise while PMTs were not (see also Beyer et al. [17] or similar results in another type of conflict task). This MT reduction might be due to an increase of global muscle tone at exercise, likely driven by the higher arousal state usually induced by exercise ([36] for an illustration of arousing effects of exercise); note that, conversely, at lower arousal states generated by one night sleep deprivation, MTs are lengthened while PMT are not [37]. If exercise induces a higher basal muscle tone, once motor units are recruited, the response threshold is reached earlier. In the exercise condition, the increase of HF/LF ratio in both response-involved and non-involved first interosseous dorsalis lends support this explanation. In any event, regardless of the reason why MTs are shortened at exercise, this shortening could explain the concomitant reduction of the correction ratio, as we will develop in the following.
As indicated in the results section, the fact that exercise had no influence on the incorrect activation rate but, nevertheless, decreased the correction ratio, manifests a pure trade-off between partial error and error rates at exercise. How could such a trade-off be explained? All mental operations take time [38] detection and inhibition of an incorrect activation likely need a certain delay too [8]. When this delay is longer than the motor time, it can be reasoned that there is no time enough for detection, inhibition and correction processes to fully develop; in this case, an incorrect activation would necessarily turn into a full-blown error. In other words, a certain proportion of incorrect activations that would likely be detected, inhibited and corrected on time if initiated in a control condition (with long enough MTs), would not find enough time, at exercise, for allowing the (otherwise unimpaired) detection, inhibition and correction processes to take place; this simple reduction of MTs would “mechanically” generate the observed trade-off between partial errors and errors. This interpretation is supported in the present experiment by the existence of an inter-subject correlation between the MT and the correction ratio. If this explanation is valid, other experimental conditions which generate shorter MTs should also generate smaller correction ratios.
As compared to accuracy instructions, MTs are strongly reduced to the point of accounting for 20% of the RTs reduction observed under speed instructions [39] still under speed instructions, the correction ratio is decreased [40]. Spieser et al. [39] reasoned that “… speeding up response execution reduces the time available for a correction, thereby directly impacting correction.” (page 956).
Dopaminergic treatment in Parkinson’s disease generates a speed-accuracy trade-off (SATO) in patients [41]. The shortening of the RTs in the “on” as compared to the “off” condition was completely explained by a reduction of MTs , PMTs being spared by the dopaminergic treatment. Moreover, although the correction ratio decreased under medication, the incorrect activation rate remained unaffected [41]. Therefore, the decreased correction ratio in the “on” as compared to the “off” condition was completely explained by a trade-off between partial errors and errors, at the expense of partial errors. These effects perfectly mimic the results observed here at exercise.
Therefore, it seems that, although the correction of incorrect activations decreases at exercise, the underlying action monitoring mechanisms responsible for this correction are not impaired by exercise. On the contrary, correction processes likely function normally but they encounter peripheral unfavorable working conditions to be fully efficient: the shorter MTs do not let enough time for the correction processes to fully develop. Put differently and counter intuitively, although not impaired, correction processes are less efficient at exercise.
What about the effect of heavy exercise on the correction ratio already reported by Schmit et al., [19]? Could it be explained by the same processes as those proposed here for moderate exercise, SATO or levodopa medication in Parkinson’s disease? The answer is not straightforward mainly because the intensity of the exercise for which the authors report a reduction of the correction ratio is not comparable to the intensity of the exercise used in the present experiment Therefore, one cannot directly apply our interpretations of the effects of sustained exercise on the correction ratio, to the effects reported by Schmit et al. [19], close to exhaustion, even though these effects look quite similar. Now, the absence of influence of exercise on RTs before exhaustion, reported by Schmit et al. [19], did not indicate any absence of effects of this exercise on MTs since the authors defined their RT measure as the delay separating the presentation of the stimulus from EMG onset; this measure corresponds to the PMT as defined in the present experiment. Therefore, one cannot exclude that the reduction of the correction ratio was due (at least in part) to a reduction of the MTs. Table 2 of Schmit et al. [19] suggests that exhaustion increased the rate of incorrect activations (although this was not the case for partial error rates). If this were the case, it is possible that for this type of exercise, a true impairment of correction process appeared just before exhaustion. However, further investigation is needed to answer this question.
The present results go beyond the strict domain of the effects of exercise on cognitive control. In line with Servant et al. [1] or Spieser et al. [39], and contrary to what is often assumed, the present data show that the most peripheral motor processes are not immune from the influence of upstream processes. Moreover, it seems that, when subjects process sensori-motor information under time pressure, any condition which reduces the motor time is liable to reducing the efficiency of the correction processes. Therefore, observing a drop in the efficiency of cognitive control processes does not necessarily imply that these processes are deficient.
Given the association of reduced RTs and increased error rate at exercise or under dopa therapy in Parkinson’s disease, the behavioral effects reported here or the behavioral effects of dopa therapy in Parkinson’s disease reported by Fluchère et al. [41], can factually be described as a SATO. However, this SATO seems only to be due to a lack of time for correction operations to take place. Therefore, SATO, may correspond to a mixture of effects: insufficient time let to process information leading to (premature) incorrect decisions, insufficient time to perform (otherwise correct) correction operations, or both. Identification of partial errors thanks to EMG recordings allows disentangling these possible sources of SATO.
More generally, we have argued earlier that there are two (not mutually exclusive) ways to deteriorate performance in terms of accuracy: decreasing the efficiency of the information processing chain or decreasing the efficiency of the action monitoring system [43]. Now, the present data, the effects of speed/accuracy instructions [39,40] or the effects of dopaminergic therapy [41], indicate that the action monitoring system efficiency can be reduced although it is operating correctly. As a consequence, one can conclude that there are not two but (at least) three (not mutually exclusive) ways to deteriorate performance in terms of accuracy: decreasing the efficiency of the information processing chain, hampering action monitoring operations or generating conditions that render inoperative these (otherwise correct) operations.
This work is supported by the French Army Health Service (project number: 2014PPRC05).
All authors claim that there are no conflicts of interest.
- Servant M, White C, Montagnini A, Burle B (2015) Using covert response activation to test latent assumptions of formal decision-making models in humans. The Journal of Neuroscience 35: 10371-10385. [Crossref]
- Botwinick J, Thompson LW (1966) Premotor and motor components of reaction time. Journal of Experimental Psychology 71: 9-15. [Crossref]
- Rabbitt PM (1966) Errors and error correction in choice-response tasks. Journal of Experimental Psychology 71: 264-272.
- Eriksen CW (1985) An electromyographic examination of response competition. Bulletin of the Psychonomic Society 23: 165-168.
- Smid HG (1990) Selective response activation can begin before stimulus recognition is complete: A psychophysiological and error analysis of continuous flow. Acta psychologica 74: 169-210. [Crossref]
- Scheffers MK (1996) Event‐related brain potentials and error‐related processing: An analysis of incorrect responses to go and no‐go stimuli. Psychophysiology 33: 42-53. [Crossref]
- Maruo Y (2017) The effect of monetary punishment on error evaluation in a Go/No-go task. International Journal of Psychophysiology 120: 54-59. [Crossref]
- Hasbroucq T (1999) Effect of the irrelevant location of the response signal on choice reaction time: An electromyographic study in humans. Psychophysiology 36: 522-526.
- Allain S (2009) Sequential adjustments before and after partial errors. Psychonomic Bulletin &Review 16: 356-362.
- Ficarella SC (2019) Becoming aware of subliminal responses: an EEG/EMG study on partial error detection and correction in humans. Cortex 120: 443-456.
- Burle B (2002) Executive control in the simon effect: An electromyographic and distributional analysis. Psychological Research 66: 324-336.
- Ratcliff R, Smith PL (2004) A comparison of sequential sampling models for two-choice reaction time. Psychological Review 111: 333-367.
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD (2006) The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychological Review 113: 700-765.
- Davranche, K, Burle B, Audiffren M, Hasbroucq T (2006) Physical exercise facilitates motor processes in simple reaction time performance: an electromyographic analysis. Neuroscience Letters 396: 54-56.
- Davranche, K, Burle B, Audiffren M, Hasbroucq T (2005) Information processing during physical exercise: a chronometric and electromyographic study. Experimental Brain Research 165: 532-540.
- Audiffren M, Tomporowski PD, Zagrodnik J (2008) Acute aerobic exercise and information processing: energizing motor processes during a choice reaction time task. ActaPsychologica 129: 410-419.
- Beyer KS, Stout JR, Fukuda DH, Jajtner AR, Townsend JR, et al. (2017) Impact of polyphenol supplementation on acute and chronic response to resistance training. Journal of Strength and Conditioning Research 31: 2945-2954. [Crossref]
- Cooper A (1973) An analysis of the possible source of contractile forces in striated muscle. Journal of Theoretical Biology 42: 545-562.
- Schmit C, Davranche K, Easthope CS, Colson SS, Brisswalter J, et al. (2015) Pushing to the limits: The dynamics of cognitive control during exhausting exercise. Neuropsychologia 68: 71-81.
- Davranche K, Brisswalter J, Radel R (2015) Where are the limits of the effects of exercise intensity on cognitive control ? Journal of Sport and Health Science 4: 56-63
- Kornblum S, Hasbroucq T, Osman A (1990) Dimensional overlap: Cognitive basis for stimulus-response compatibility--a model and taxonomy. Psychological Review 97: 253-270.
- Ridderinkhof KR (2002) Activation and suppression in conflict tasks: empirical classification through distributional analyses. In: Prinz, W., Hommel, B. (Eds.) Common mechanisms in perception and action. Attention and performance. Oxford University Press, Oxford 494-519.
- van den Wildenberg WP, Wylie SA, Forstmann BU, Burle B, Hasbroucq T (2010) To Head or to Heed? Beyond the surface of selective action inhibition: A review. Frontiers in Human Neuroscience 4: 222.
- Simon JR (1990) The effects of an irrelevant directional cue on human information processing. In: Reeve, R.W.P.T.G. (Ed.) Stimulus-response Compatibility. Elsevier Science Publishers/North-Holland, pp. 31-86.
- Hommel B (2011) Attention and spatial stimulus coding in the simon task: A rejoinder to van Der Lubbe and Abrahamse (2010) ActaPsychologica 136: 265-268.
- De Jong R, Liang CC, Lauber E (1994) Conditional and unconditional automaticity: A dual-process model of effects of spatial stimulus-response correspondence. Journal of Experimental Psychology. Human Perception and Performance 20: 731-750.
- Kornblum S (1994) The way irrelevant dimensions Are processed depends on what they overlap with: the case of stroop- and simon-like stimuli. Psychological Research 56: 130-135.
- Proctor RW (1995) Activation of response codes by relevant and irrelevant stimulus information. ActaPsychologica 90: 275-286.
- Burle B, Bonnet M (1999) What’s an internal clock for? From temporal information processing to temporal processing of information. Behavioural Processes 45: 59-72.
- Tomberg, C., and Caramia M. D. (1991) prime mover muscle in finger lift or finger flexion reaction times: identification with transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology 81: 319-322.
- Kukulka CG, Clamann HP (1981) Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Research 219: 45-55.
- Christensen H (1986) Muscle activity and fatigue in the shoulder muscles of assembly-plant employees. Scandinavian Journal of Work, Environment and Health 12: 582-587.
- Staude GH (2001) Precise onset detection of human motor responses using a whitening filter and the log-likelihood-ratio test. IEEE Transactions on Bio-Medical Engineering 48: 1292-1305.
- Ratcliff R (1979) Group reaction time distributions and an analysis of distribution statistics. Psychological Bulletin 86: 446-461.
- Vincent SB (1912) The function of vibrossae in the behavior of the white rate. Behavioral Monographs 1: 1-181.
- Servant M, Gajdos T, Davranche K (2018) ELF: A new measure of response capture. Psychonomic Bulletin and Review 25: 539-547.
- Barba A, Padilla F, Luque-Casado A, Sanabria D, Correa Á (2018) The role of exercise-induced arousal and exposure to blue-enriched lighting on vigilance. Frontiers in Human Neuroscience 12: 499.
- Ramdani C, Carbonnell L, Rabat A, Meckler C, Burle B (2013) Sleep deprivation affects the sensitivity of proactive and reactive action monitoring: A behavioural and ERP analysis. Biological Psychology 93: 237-245.
- Donders FC (1868) On the speed of mental processes. ArchivesNeerlandaise 3: 269-317.
- Spieser L, Servant M, Hasbroucq T, Burle B (2017) Beyond decision! Motor contribution to speed-accuracy trade-off in decision-making. Psychonomic Bulletin and Review 24: 950-956.
- Burle B, Spieser L, Servant M, Hasbroucq T (2014) Distributional reaction time properties in the Eriksen task: marked differences or hidden similarities with the Simon task? Psychon Bull Rev 21: 1003-1010.
- Fluchère F, Burle B, Vidal F, van den Wildenberg W, Witjas T (2018) Subthalamic nucleus stimulation, dopaminergic treatment and impulsivity in parkinson’s disease. Neuropsychologia 117: 167-177. [Crossref]
- Hasbroucq T, Tandonnet C, Micallef-Roll J, Blin O, Possamaï CA (2003) An electromyographic analysis of the effect of levodopa on the response time of healthy subjects. Psychopharmacology 165: 313-316.
- Vidal F, Meckler C, Hasbroucq T (2015) Basics for sensorimotor information processing: some implications for learning. Frontiers in Psychology 6: 33.








