Abstract
Background: Social isolation (SI) induces alterations in brain development, leading to behavioral impairments similar to schizophrenia-like phenotypes. A developmental model of schizophrenia is achieved by SI of weanling rats and mice. This study conducts a systematic review and meta-analysis to evaluate the behavioral consequences of SI on rodents comprehensively and explore potential heterogeneous factors reflective of schizophrenia-related phenotypes.
Methods: Employing a meticulous search strategy across PubMed, Web of Science, Embase, Wan fang and CNKI, this study adheres to PRISMA guidelines. The analysis includes studies examining the impact of post-weaning social isolation rearing effects on behavioral outcomes, such as Elevated Plus Maze (EPM) performance, Open Field Test (OFT), Pre-pulse Inhibition (PPI) and Morris Water Maze (MWM). The study protocol is registered with PROSPERO, number CRD420250610010.
Results: A total of 43 articles were included. SI rodents in the EPM group exhibit a Mean Difference (MD) of -0.29 (95% CI: (-0.58, -0.00)) in adulthood. SI rodents show a significant overall effect on anxiety-like behavior, reduced open-arm exploration. Notable inter-study heterogeneity was observed. SI significantly increased locomotor activity in OFT. Adulthood results show a MD of 1.53 (95% CI: (1.12, 1.93)). However, studies showed substantial heterogeneity. In mice, a MD showing average reductions of -0.59 (95% CI: (−1.18, -0.00)) for PPI (66-70 low), -0.16 (95% CI: (−0.63, 0.31)) for PPI (71-76 medium) and -0.86 (95% CI = (−1.32, −0.40)) for PPI (77- high) with SI when compared with healthy controls. The heterogeneity across studies was low. In rats, all pre-pulse intensities showed significant PPI reductions in SI groups (p < 0.0001 for all), though substantial heterogeneity was observed, possibly linked to strain differences (e.g., Wistar vs. Sprague-Dawley) and variations in isolation onset age. The overall effect of SI on MWM performance was statistically significant, shows a mean difference of 0.97 (95% CI: (0.31, 1.62)), indicating altered spatial learning/memory. However, extreme inter-study heterogeneity was present. The overall data suggested that SI had a large effect on exacerbating schizophrenia-like behaviors, but high heterogeneity was found in several behavioral analyses.
Conclusion: This systematic review and meta-analysis offer a comprehensive overview of behavioral consequences linked to post-weaning social isolation rearing in rodents. The data suggested that SI had a significant overall effect on exacerbating schizophrenia-like behaviors in animal models. More high-quality studies with longer follow-up times are needed to strengthen this evidence. It has promoted the understanding of the impact of SI on neurodevelopment and put forward suggestions for future research in related fields.
Keywords
schizophrenia, rodent models, social isolation, behavioral analysis
Introduction
The prevalence of schizophrenia is about 1% worldwide, and patients often suffer from disturbances in perception, thinking, emotion, and behavior and incoordination of mental activities [1]. The psychosocial hypothesis is a widely recognized etiology of schizophrenia, which suggests that the onset of schizophrenia occurs at a critical time during early development. It is believed that environmental stresses at critical times in early development alter brain development leading to behavioral deficits in adulthood. The social isolation (SI) model is a representative paradigm of the psychosocial hypothesis, in which mice are isolated immediately after weaning (P21) to perturb neurodevelopmental trajectories, leading to persistent behavioral deficits in adulthood. Therefore, an in-depth study of the SI model is of great significance for understanding the etiology of schizophrenia, screening of therapeutic drugs, and intervention measures [2].
While modeling the positive symptoms of schizophrenia (hallucinations and delusions) in animals is inherently difficult, SI rearing effectively models core negative and cognitive symptoms, including impairments in cognition, memory, emotion, and social interaction. Specifically, SI rearing in rodents consistently induces a range of alterations such as increased anxiety- and depression-like behaviors, deficits in sensorimotor gating, and impaired learning and memory [3]. This meta-analysis will quantitatively synthesize the effects of post-weaning social isolation rearing in rodents on key schizophrenia-relevant behavioral domains. We selected four well-established behavioral tests: the Elevated Plus Maze (EPM) and Open Field Test (OFT) to assess anxiety and exploratory behavior/ activity, Pre-pulse Inhibition (PPI) to measure sensorimotor gating, and the Morris Water Maze (MWM) to evaluate spatial learning and memory.
Materials and methods
Search strategy
Five online databases (PubMed, Web of Science, Embase, Wanfang, CNKI) were systematically searched until May 30, 2025 to identify experimental studies investigating schizophrenia-like behaviors induced by SI in rodent models. The search strategy focused on three key domains: social isolation (intervention), schizophrenia-like behavior (outcome), and rats/mice (population). We using the following search:
((("Social Isolation"[Mesh]) OR ((((Isolation, Social[Title/Abstract]) OR (Social Exclusion[Title/Abstract])) OR (Exclusion, Social[Title/Abstract])) OR (Social Exclusions[Title/Abstract]))) AND (("Schizophrenia"[Mesh]) OR (((((Schizophrenias[Title/Abstract]) OR (Schizophrenic Disorders[Title/Abstract])) OR (Disorder, Schizophrenic[Title/Abstract])) OR (Schizophrenic Disorder[Title/Abstract])) OR (SCZ[Title/Abstract])))) AND (("Behavior"[Mesh]) OR (behaviors[Title/Abstract]))
After excluding duplicate references, we screened titles and abstracts to identify potentially relevant articles that fit the inclusion criteria. The titles and abstracts of the studies obtained through the search were examined by two reviewers in order to determine article inclusion. The next step is to conduct a full-text analysis of the remaining articles.
Data extraction
Experimental studies that assessed the effects of schizophrenia-like behavior in rodents were considered eligible. The following inclusion criteria were used:
Article type: Experimental studies.
Participants: Rodents. Genetically modified mouse were also excluded.
Intervention: social isolation rearing as an intervention.
Comparison: Except for social isolation rearing, the rest of the interventions (room, handlers, diet, light cycle, and enrichment provision) were the same in the experimental group and the control group.
Outcomes: Results from at least one of the experiments involving EPM, OFT, PPI or MWM.
There was no language, publication date or other restrictions.
In the first selection phase (title and abstract), exclusion criteria were applied in the following order: (1) reviews/ theoretical articles (2) wrong population (not rodents) and (3) wrong intervention (add Surgical and pharmacological methods). In the second selection phase (full texts), exclusion criteria were applied in the following order: (1) full text not available (2) no control group or condition (3) no OFT, EPM, PPI or MWM experiments (4) no uniform experimental data (5) no appropriate behavioral assessment.
Data was extracted from each article by Wanqi Jin and Minyue Zhang. To resolve discrepancies, a third reviewer Yumu Guo was consulted. Comprehensive information was extracted from each article, encompassing details such as authors, title, sample size, species, strain, and age of the subjects, types of outcomes tested, as well as control animals. For every comparison, the numerical variable used to evaluate the behavior of animals was meticulously extracted in the form of mean ± standard deviation (SD). When these data were available only in graphical form, the program WebPlotDigitizer [4] was utilized to convert graphically represented data into numerical values using the distance measurement function [5]. A detailed information with all variables is available in Tables 1-4. For studies disclosing standard error of the mean, the SD was calculated by dividing it by the square root of the sample size.
Table 1. Studies on EPM
Table 2. Studies on OFT
Table 3. Studies on PPI
Table 4. Studies on MWM
Statistical analysis
All statistical analyses were performed using Cochrane Review Manager 5.3. A random-effects model was selected as the primary meta-analytic approach due to anticipated heterogeneity across studies. Meta-analyses were conducted only when ≥ 3 studies reported comparable outcome data. All primary results on the four outcomes of interest, OFT, EPM, PPI and MWM are presented for the overall sample (stratified by species). Hedge’s g was used as the pooled measurement of effect size as it is preferred over Cohen’s d for small samples (which are common in animal studies). Cochran’s Q test and the I² index were used to test heterogeneity between studies. Data were presented in Forest plots as effect size ± 95% confidence interval (CI). Results were considered significant when CIs did not contain zero, associated with p < 0.05.
Results
Search results 1745 articles were identified for consideration in the present meta-analysis. 41 studies were eligible for inclusion in this meta-analysis (Tables 1-4). Of the 41 included studies: 10 assessed anxiety-like behavior using EPM, 6 evaluated locomotor activity via OFT, 31 measured sensorimotor gating with PPI, 5 examined spatial cognition through the MWM. Each article underwent a detailed data extraction process (Figure 1), as described in the methodology section.
Figure 1. PRISMA flow-diagram of the study selection process for the meta-analysis
Outcome
EPM: In the EPM trial, the association between social isolation rodents and anxiety in schizophrenia-like behaviors was described in studies using EPMs to examine this issue (Figure 2). A total of 9 articles met the inclusion criteria and were included in EPM [6-14].
The overall effect of social isolation rearing on EPM was significant (95% confidence interval =-0.29 (-0.58,-0.00), Z=1.98, p=0.05), and inter-study heterogeneity was significant (I2=79%, Chi2=37.95, df=8).Suggesting that the association between social isolation and increased EPM performance was statistically significant, although this reduced schizophrenia-like behaviors was only significant in mice (95% confidence interval=-0.41 (-0.78, -0.03) of mice) (Figure 2).

Figure 2. EPM forest plot
OFT: Six studies [15-20] quantified the effects of SI on exploratory behavior using the OFT (Figure 3). SI induced significant hyperlocomotion (MD=1.53, 95% Cl (1.12, 1.93); Z=7.43, p < 0.00001) with substantial between-study heterogeneity (I2=91%, χ2=57.01, df=5, p < 0.00001).
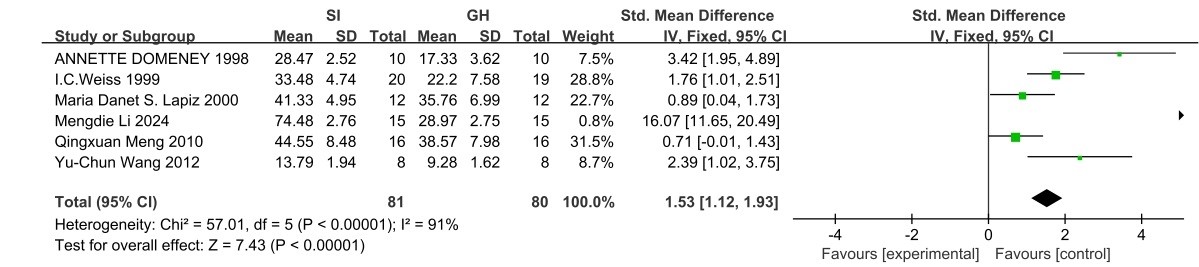
Figure 3. OFT forest plot
PPI: In the PPI trial, 31articles were included that described the use of PPI to examine the effects of social isolation rearing on cognitive and sensory impairments in schizophrenic behavior (Figures 4 and 5) [13,15,16,19,21-43].
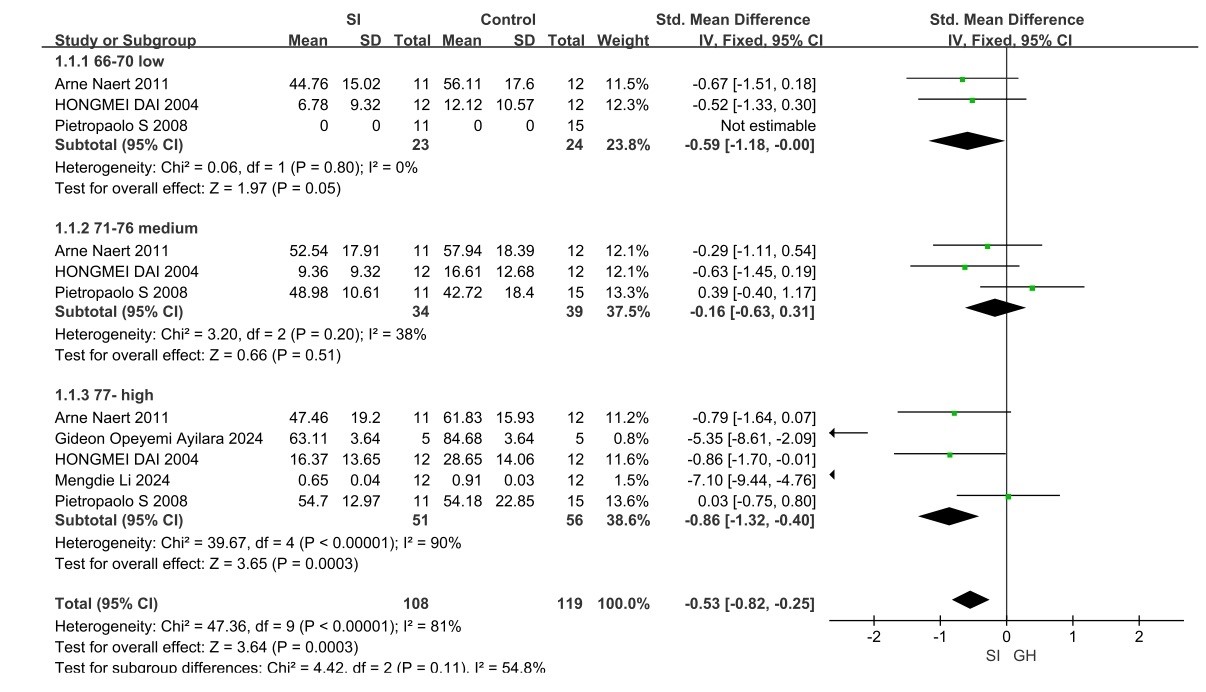
Figure 4. PPI forest plot for mice
In the mice group, it (Figure 4) shows the forest plot for PPI(66-70 low), PPI(71-76 medium) and PPI(77- high) SDs with 95% confidence intervals and a summary mean difference showing average reductions of PPI(66-70 low) (95% CI=-0.59(−1.18, -0.00), p=0.05) , PPI(71-76 medium) (95% CI=-0.16(−0.63, 0.31), p=0.51) and PPI(77- high) (95% CI=-0.86(−1.32, −0.40), p=0.0003) in mice with SI when compared with healthy controls. The heterogeneity across studies was low for both PPI (66-70 low) (I2=0%, Chi2=0.06), PPI (71-76 medium) (I2=38%, Chi2=3.20) and PPI (77- high)) (I2=90%, Chi2=39.67).
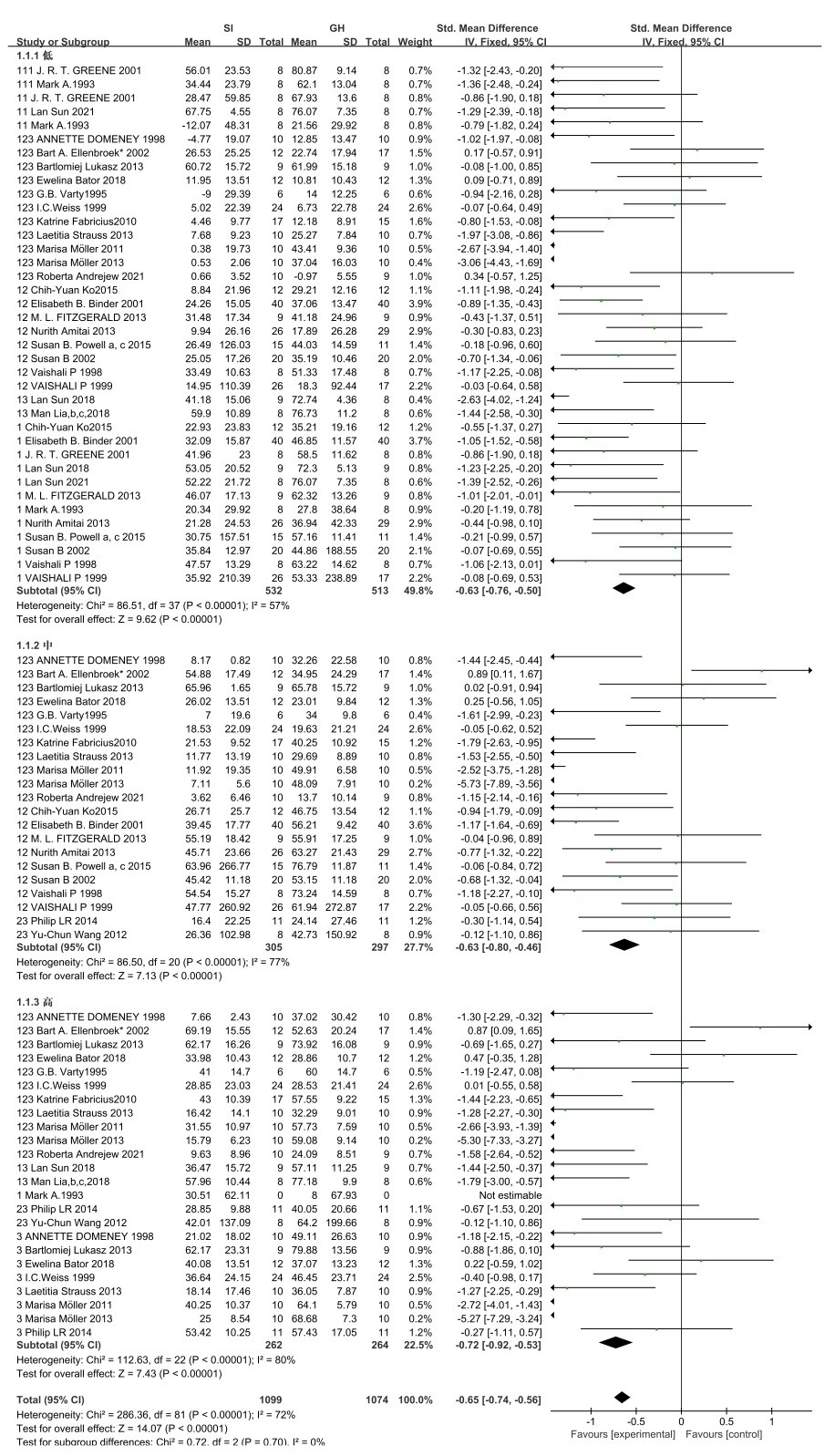
Figure 5. PPI forest plot for rats
In the rats group, it (Figure 5) shows the forest plot for PPI(66-70 low), PPI(71-76 medium) and PPI(77- high) SDs with 95% confidence intervals and a summary mean difference showing average reductions of PPI(66-70 low) (95% CI=-0.63(-0.76, -0.05), p<0.00001) PPI(71-76 medium) (95% CI=-0.63(-0.80, 0.46), p < 0.00001) and PPI(77- high) (95% CI=-0.72(-0.92, -0.53), p < 0.0001) with SI when compared with healthy controls. The heterogeneity across studies was substantial for both PPI (66-70 low) (I2 =57%, Chi2=86.5, df=37), PPI (71-76 medium) (I2=77%, Chi2=86.50, df=20) and PPI (77- high) (I2=80%, Chi2=112.63, df=22).
MWM: The MWM experiment, which included five articles ( 18,20,32,44,45] with a total of 96 subjects, described the use of the MWM to examine the effects of social isolation rearing on learning and memory function in schizophrenia-like behavior (Figure 6). In MWM, the overall effect of social isolation rearing was significant (95% confidence interval=0.97 (0.31, 1.62), Z=2.90, p=0.004), and inter-study heterogeneity was significant (I2=96%, χ2=91.55, df=4).
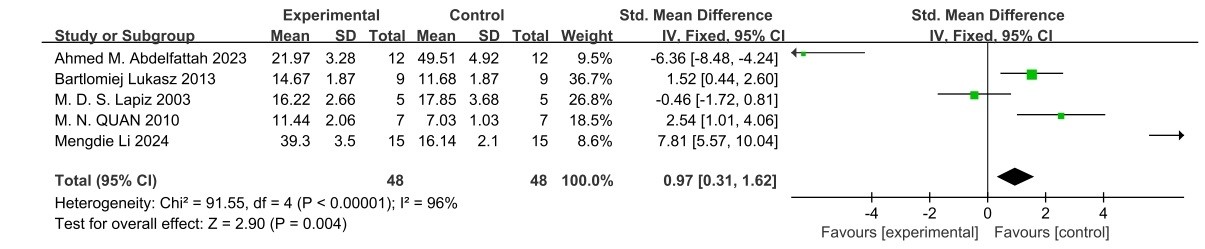
Figure 6. MWM forest plot
Discussion
Meta-analyses of animal data have become increasingly relevant over the last few years, which not only help researchers understand the pathogenesis of diseases, but also provide a testing platform for new treatment programs. To the best of our knowledge, this study represents the first systematic review and meta-analysis investigating the impact of social isolation on schizophrenia-like behavior in rodents.
In recent years, some studies have investigated the effect of social isolation rearing on the rodents of schizophrenia-like behavior have reported various effects, which in general are promising. But we have not been able to state which are the overall effects of social isolation rearing on schizophrenia-like behavior and in which social isolation rearing in animal models is able to produce an unambiguous increase in schizophrenia-like behavior.
We found that the social isolation rearing is strongly associated with anxiety behavior, exploratory behavior, cognitive and sensory impairments and learning and memory function (i.e., The main behavioral symptoms of schizophrenia). These schizophrenic-like behaviors tended to increase in the social isolation rearing group compared with control animals (Figures 2-5). The publication bias was assessed using a funnel plot, indicating marginal effect of publication bias mostly due to small sample size or inadequate reports of negative data. However, most studies were distributed in a funnel-shaped area of the plot.
However, Findings were consistent in sensitivity analyses but there was noted to be a large amount of statistical heterogeneity. This suggests that different research groups have trouble reproducing schizophrenia-like behavior (Figures 3-6). Especially, Matar, et al. [46] and colleagues highlighted that a heterogeneous response may confirm the validity of animal studies because humans do not clearly respond homogeneously to potentially traumatic experiences [46]. In the present study, the overall outcome of the high heterogeneity data had a large effect on exacerbating schizophrenia-like behavior, possibly related to differences in social isolation parenting time and inconsistent behavioral experimental operations. While some studies reported schizophrenia-like behaviors results induced by social isolation rearing, others reported increased schizophrenia-like behaviors. Therefore, we needed to explore the sources of heterogeneity and obtained more accurate results. Although, Injection behavior had widely varying effects on biological processes and behavior, we included them considering the fact that most articles contained them and the comprehensiveness of the results.
Due to the limited data sample, we only performed interspecific analyses of EPM and PPI. We only found rodent strain differences in the anxiety are prominent in the literature in EPM. In this meta-analysis, it was evident that there was no significant statistical significance in schizophrenic behavior between the mice after social isolation rearing and the control group (p > 0.05). In the PPI experiment, there was no significant difference between rats and mice. In the subgroup analysis of PPI, compared with the experimental group and the control group, there is a higher difference in the decibels in the middle, which indicates that under the decibels above 66-70 dB and 77 dB, social isolation rearing has A greater impact on cognitive and sensory impairments (A type of schizophrenic behavior). Altogether, discrepancies among these studies may be a result of varying isolation procedures (different onsets and durations), differing in experimental conditions (more anxiogenic or less), differing in age when tested, as well as strain and sex differences [47]. A number of articles refer to schizophrenia-like behaviors differences after gender is bred for social separation. Some study findings are contrary to a similar study using Swiss–Kunming mice, which revealed a male-preferentially decreased despair behavior in isolated groups. Another study found that male but not female-isolated-reared rats exhibited a higher percentage of time in open arms of EPM compared with their group-reared controls [47].
A number of limitations of the research included in this Meta-analysis should be acknowledged. Firstly, due to the limited number of publications, the funnel plot shows bias, suggesting that more high-quality studies should be included. Secondly, although the database has been carefully and comprehensively searched, there are still few studies selected for each behavioral blood type, which can lead to bias. Aside from the abovementioned speculations, several limitations should be noted. The data derived using digital software were different from the actual study results, so there was a certain degree of data error. In addition, our meta-analysis checked only rodents and it was unknown what social isolation rearing did in other animals. Future research should target such limitations.
Conclusion
This meta-analysis confirms that post-weaning social isolation rearing induces robust schizophrenia-like behavioral deficits in rodents, manifested as hyperactivity in OFT, anxiety-like responses in the EPM, and sensorimotor gating impairments in PPI. The observed effects demonstrate significant strain-dependent heterogeneity, underscoring the necessity for standardized protocols with extended isolation durations ( > 12 weeks) and multi-domain behavioral assessments in future studies. Researchers should prioritize isolation paradigms aligned with specific schizophrenia endophenotypes to optimize translational validity.
CRediT authorship contribution statement
Minyue Zhang: Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Wanqi Jin: Writing–review & editing, Writing–original draft, Visualization, Software, Resources, Methodology, Investigation, Formal analysis, Data curation. Yumu Guo: Supervision, Data curation. Chunyue Huo: Validation, Supervision, Project administration, Conceptualization.
Conflicts of interest
The authors declare that no actual or potential conflicts of interest exist, including any financial, personal, or other relationships with other people or organizations.
Funding
This study was supported by the Research Incubation Fund of Capital Medical University Yanjing Medical College (20kyqd01). The funding agency has no role in the design, analysis, interpretation, or writing of this study.
Acknowledgment
We would like to thank the researchers who provided us with data of their original studies.
Availability of data and materials
The datasets generated and analyzed during the current study are publicly available.
References
- Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar AV, et al. (2016) The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry 77: 764-771. [Crossref]
- Winship IR, Dursun SM, Baker GB, Balista PA, Kandratavicius L, et al. (2019) An overview of animal models related to schizophrenia. Can J Psychiatry 64: 5-10. [Crossref]
- Matsumoto K, Fujiwara H, Araki R, Yabe T(2011) Post-weaning social isolation of mice: A putative animal model of developmental disorders. J Pharmacol Sci 141: 111-118. [Crossref]
- Drevon D, Fursa SR, Malcolm AL (2017) Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif 41: 323-339. [Crossref]
- Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, et al. (2018) A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med 52: 376-384. [Crossref]
- Molina-Hernandez MP, Tellez-Alcantara, Perez-Garcia J (2001). Isolation rearing induced fear-like behavior without affecting learning abilities of wistar rats. Prog Neuropsychopharmacol Biol Psychiatry 25: 1111-1123. [Crossref]
- Pietropaolo S, Singer P, Feldon J, Yee BK (2008) The postweaning social isolation in C57BL/6 mice: Preferential vulnerability in the male sex. Psychopharmacology (Berl) 197: 613-628. [Crossref]
- Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, et al. (2009) Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res 202: 114-121. [Crossref]
- Sciolino NR, Bortolato M, Eisenstein SA, Fu J, Oveisi FA, et al. (2010) Social isolation and chronic handling alter endocannabinoid signaling and behavioral reactivity to context in adult rats. Neuroscience 168: 371-386. [Crossref]
- Simpson SM, Menard JL, Reynolds JN, Beninger RJ (2010) Post-weaning social isolation increases activity in a novel environment but decreases defensive burying and subchronic MK-801 enhances the activity but not the burying effect in rats. Pharmacol Biochem Behav 95: 72-79. [Crossref]
- Bouet V, Lecrux B, Tran G, Freret T (2011) Effect of pre-versus post-weaning environmental disturbances on social behaviour in mice. Neurosci Lett 488: 221-224. [Crossref]
- Huang Q, Zhou Y, Liu LY (2017) Effect of post-weaning isolation on anxiety-and depressive-like behaviors of C57BL/6J mice. Exp Brain Res 235: 2893-2899. [Crossref]
- Sun L, Min L, Li M, Shao F, Wang W, et al. (2018) Transcriptomic analysis reveals oxidative phosphorylation activation in an adolescent social isolation rat model. Brain Res Bull 142: 304-312. [Crossref]
- Shangase KB, Luvuno M, Mabandla M (2025) Effects of combined postweaning social isolation and ketamine administration on schizophrenia-like behaviour in male sprague dawley rats. Behav Brain Res 476: 115214. [Crossref]
- Domeney A, Feldon J (1998) The disruption of prepulse inhibition by social isolation in the wistar rat: How robust is the effect?. Pharmacol Biochem Behav 59: 883-890. [Crossref]
- Weiss IC, Feldon J, Domeney AM, Buell MR, Geyer MA, et al. (1999) Isolation rearing-induced disruption of prepulse inhibition: Further evidence for fragility of the response. Behav Pharmacol 10: 139-149.[Crossref]
- Lapiz MD, Mateo Y, Parker T, Marsden C (2000) Effects of noradrenaline depletion in the brain on response to novelty in isolation-reared rats. Psychopharmacology 152: 312-320. [Crossref]
- Meng Q, Li N, Han N, Shao F, Wang W, et al. (2010) Peri-adolescence isolation rearing alters social behavior and nociception in rats. Neurosci Lett 480: 25-29.[Crossref]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ, et al. (2012) Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct 217: 337-351. [Crossref]
- Li M, Liu Y, Sun M, Yang Y, Zhang L, et al. (2024) SEP‐363856 exerts neuroprotection through the PI3K/AKT/GSK‐3β signaling pathway in a dual‐hit neurodevelopmental model of schizophrenia‐like mice. Drug Dev Res 85: e22225. [Crossref]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW (1993) Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry 34: 361-372. [Crossref]
- Varty GB, Higgins GA (1995) Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology (Berl) 122: 15-26. [Crossref]
- Bakshi VP, Swerdlow NR, Braff DL, Geyer MA (1998) Reversal of isolation rearing-induced deficits in prepulse inhibition by seroquel and olanzapine. Biol Psychiatry 43: 436-445. [Crossref]
- Bakshi VP, Geyer MA (1999) Ontogeny of isolation rearing-induced deficits in sensorimotor gating in rats. Physiol Behav 67: 385-392. [Crossref]
- Binder EB, Kinkead B, Owens MJ, Kilts CD, Nemeroff CB, et al. (2001) Enhanced neurotensin neurotransmission is involved in the clinically relevant behavioral effects of antipsychotic drugs: Evidence from animal models of sensorimotor gating. J Neurosci 21: 601-608. [Crossref]
- Greene JR, Kerkhoff JE, Guiver L, Totterdell S (2001) Structural and functional abnormalities of the hippocampal formation in rats with environmentally induced reductions in prepulse inhibition of acoustic startle. Neuroscience 103: 315-323. [Crossref]
- Ellenbroek BA, Cools AR (2002) Early maternal deprivation and prepulse inhibition: The role of the postdeprivation environment. Pharmacol Biochem Behav 73: 177-184. [Crossref]
- Powell SB, Swerdlow NR, Pitcher LK, Geyer MA (2002) Isolation rearing-induced deficits in prepulse inhibition and locomotor habituation are not potentiated by water deprivation. Physiol Behav 77: 55-64. [Crossref]
- Fabricius K, Helboe L, Fink-Jensen A, Wörtwein G, Steiniger-Brach B, et al. (2010) Increased dopaminergic activity in socially isolated rats: An electrophysiological study. Neurosci Lett 482: 117-122. [Crossref]
- Möller M, Du Preez JL, Emsley R, Harvey BH (2011) Isolation rearing-induced deficits in sensorimotor gating and social interaction in rats are related to cortico-striatal oxidative stress, and reversed by sub-chronic clozapine administration. Eur Neuropsychopharmacol 21: 471-483. [Crossref]
- Fitzgerald ML, Mackie K, Pickel VM (2013) The impact of adolescent social isolation on dopamine D2 and cannabinoid CB1 receptors in the adult rat prefrontal cortex. Neuroscience 235: 40-50. [Crossref]
- Lukasz B, O’Sullivan NC, Loscher JS, Pickering M, Regan CM, et al. (2013) Peripubertal viral-like challenge and social isolation mediate overlapping but distinct effects on behaviour and brain interferon regulatory factor 7 expression in the adult wistar rat. Brain Behav Immun 27: 71-79. [Crossref]
- Möller M, Du Preez JL, Viljoen FP, Berk M, Emsley R, et al. (2013) Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav Immun 30: 156-167. [Crossref]
- Amitai N, Young JW, Higa K, Sharp RF, Geyer MA, et al. (2014) Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cogn Affect Behav Neurosci 14: 388-406. [Crossref]
- Gaskin PL, Alexander SP, Fone KC (2014) Neonatal phencyclidine administration and post-weaning social isolation as a dual-hit model of ‘schizophrenia-like’behaviour in the rat. Psychopharmacology 231: 2533-2545. [Crossref]
- Strauss L, Brink CB, Möller M, Stein DJ, Harvey BH, et al. (2014) Late-life effects of chronic methamphetamine exposure during puberty on behaviour and corticostriatal mono-amines in social isolation-reared rats. Dev Neurosci 36: 18-28. [Crossref]
- Ko CY, Liu YP (2015) Isolation rearing impaired sensorimotor gating but increased pro-inflammatory cytokines and disrupted metabolic parameters in both sexes of rats. Psychoneuroendocrinology 55: 173-183. [Crossref]
- Powell SB, Khan A, Young JW, Scott CN, Buell MR, et al. (2015) Early adolescent emergence of reversal learning impairments in isolation-reared rats. Dev Neurosci 37: 253-262. [Crossref]
- Bator E, Latusz J, Głowacka U, Radaszkiewicz A, Mudlaff K, et al. (2018) Adolescent social isolation affects schizophrenia-like behavior in the MAM-E17 model of schizophrenia. Neurotox Res 34: 305-323. [Crossref]
- Li M, Wang W, Sun L, Du W, Zhou H, et al. (2019) Chronic clozapine treatment improves the alterations of prepulse inhibition and BDNF mRNA expression in the medial prefrontal cortex that are induced by adolescent social isolation. Behav Pharmacol 30: 311-319. [Crossref]
- Andrejew R, Paim M, Moritz CE, Carreño F, Rates SM, et al. (2021) Post-weaning social isolation impairs purinergic signaling in rat brain. Neurochem Int 148: 105111. [Crossref]
- Sun L, Min L, Li M, Shao F (2021) Juvenile social isolation leads to schizophrenia-like behaviors via excess lactate production by astrocytes. Brain Res Bull 174: 240-249. [Crossref]
- Ayilara GO, Owoyele BV (2024) Bromelain prevented the onset of psychotic behaviours in social-isolation model of schizophrenia by inhibiting astrogliosis and oxido-inflammatory damage in male and female mice. Nutrire 49: 39.
- Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, et al. (2003) Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol 33: 13-29. [Crossref]
- Abdelfattah AM, Abuelezz SA, Hendawy N, Negm EA, Nawishy SA, et al. (2023) Sonic hedgehog pathway as a new target of atypical antipsychotics: Revisiting of amisulpride and aripiprazole effects in a rat model of schizophrenia. Life Sci 316: 121366. [Crossref]
- Matar MA, Zohar J, Cohen H (2013) Translationally relevant modeling of PTSD in rodents. Cell Tissue Res 354: 127-139. [Crossref]
- Lukkes JL, Engelman GH, Zelin NS, Hale MW, Lowry CA (2012) Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain Res 1443: 1-7. [Crossref]






