Breast Cancer is the most common cancer affecting women worldwide, and in Malaysia. The most common sites of metastases are bone, liver and lung [1]. Skeletal muscle involvement in various parts of the body have been reported in literature. However, the disease presentations have been varied, so long-term treatment and follow up recommendations of patients are still a debate.
We report a young woman with invasive ductal carcinoma with an enlarging metastatic nodule in the right anterior abdominal wall muscle. The immunohistochemistry of the metastatic nodule appears to differ from the primary breast tumour. The patients treatment plan and follow up is discussed.
A 36-year-old Indonesian woman presented with a left breast mass lesion, which seemed suspicious on ultrasound. She has no family history of breast cancer and was otherwise well. A mammogram ruled out multifocal disease, and a biopsy was done which confirmed a Grade 3 invasive ductal carcinoma of the breast.
A preoperative CT scan (Figure 1) was clear of metastases. A small nodule was noted on the right lower abdominal wall and was reported as a sebaceous cyst at that time.
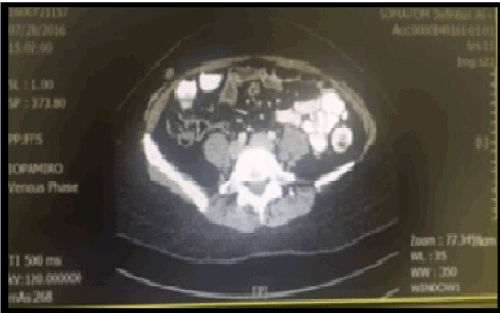
Figure 1. CT Scan image on preoperative staging (July 2016).
Her preoperative blood investigations were generally fine with normal haemoglobin, white cell count and platelets. Her liver function was normal. Her Carcino-Embryonic Antigen was less than 0.5ug/L, Ca125 was 16.1U/mL and Ca153 was 16.3 U/mL, which were all within normal limits.
She underwent a left breast wide local excision and a sentinel node biopsy uneventfully in July 2016. The final pathology report was a Grade 3 Invasive Ductal Carcinoma (Slide 1), which was 1.5cm with clear margins. Three sentinel nodes were clear of metastases. Estrogen receptors (Slide 2) and progesterone receptors (Slide 3) were negative. Her-2 was positive. Ki67 was at 60%, which is high.
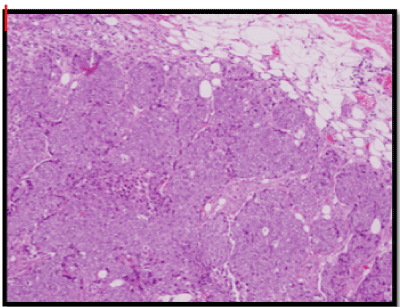
Slide 1. High Grade Invasive Ductal Carcinoma – primary breast tumour.
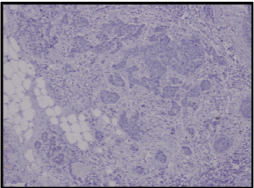
Slide 2. Estrogen receptor negative – primary breast tumour.
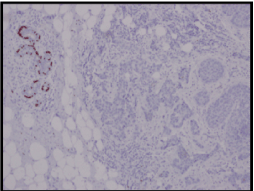
Slide 3. Progesterone receptor negative – primary breast tumour.
She underwent 6 cycles of chemotherapy (FEC) and radiotherapy to the left breast with 40Gy/15Fraction plus a 10Gy boost at a different medical centre. She was followed up regularly by the oncologist there and was apparently well. Regular ultrasounds were done, which were apparently normal.
In August 2017, she returned from Indonesia complaining of an enlarging abdominal mass lesion.
Clinically, a fairly well defined smooth mass lesion was palpable in the right lower abdomen. It appeared subcutaneous, measuring 17x19mm on bedside ultrasound. A CT scan (Figure 2) confirmed an isolated lobulated mass in the deep subcutaneous plane of the right anterolateral abdominal wall. It appeared to be infiltrating the external oblique muscle and extending into the skin. There was no evidence of metastases in other parts of the body.

Figure 2. CT Scan Image – Restaging (August 2017).
A biopsy was done which confirmed metastatic adenocarcinoma, most likely from the breast.
A wide local excision of the tumour was done (Figure3), which was reported as a 5x2x1.5cm irregular mass composed of cells arranged in fused glandular formation and cribriform pattern, consistent with metastatic adenocarcinoma (Slide 4). The tumour cells are positive for ER (Slide 5), PR (Slide 6), CK7, E-cadherin and mammaglobin. The immunoprofile is consistent with a metastatic breast carcinoma. The underlying skeletal muscle was uninvolved.
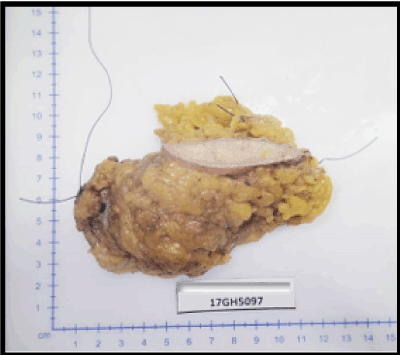
Figure 3. Gross appearance of mass removed.
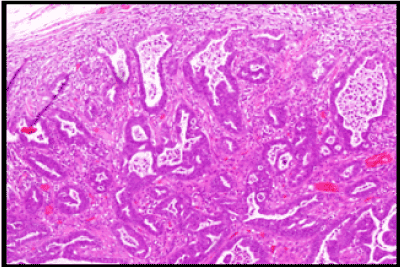
Slide 4. H&E Section of metastatic tumour.
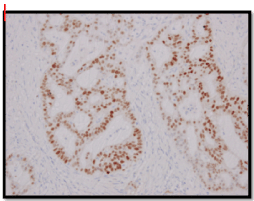
Slide 5. Section from metastatic tumour shows Estrogen Receptor positivity.
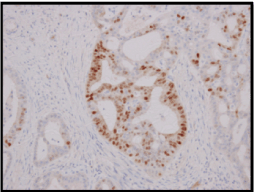
Slide 6. Section from metastatic tumour shows Progesterone Receptor positivity.
Her wound had healed well. She will be started on Tamoxifen and reviewed regularly.
Skeletal metastases from breast cancer is uncommon. There have been a few literatures reporting involvement skeletal muscles in various parts of the body. Abdominal wall muscle metastases from breast carcinoma was first reported by Ogiya et al. [2]. They reviewed 13 previously reported cases, 4 of which presented as isolated skeletal muscle metastases without other distant metastases, which is similar to this patient. However, in this patient, the metastatic lump was isolated and painless, presenting similarly to just one other patient in previous literature [3].
These metastatic lesions can be easily missed in the patients pre- and post-operative workups if not looked for carefully. Some literature has suggested the use of MRI and PET scans as the main modality to identify and confirm such metastatic lesions. Magnetic resonance imaging (MRI) is the gold standard for imaging muscle disease, and it shows features of muscle metastases that are relatively typical, consisting of well defined intramuscular masses and marked enhancement [4]. However, in developing countries, finances and resources for such investigations are often a problem and hence not routinely used.
In this patient, the initial CT scan did make a note of a small subdermal lesion but unfortunately it was dismissed as a sebaceous cyst by the radiologist. It was asymptomatic at the time, and so both the patient and the clinician did not note it. The patient did however undergo chemotherapy after surgery to the breast. Due to financial constraints, the patient had declined Herceptin therapy for the primary breast cancer at the onset. The mass, obviously recalcitrant, did not respond well as it continued to grow till the patient presented one year later with an enlarging mass in the right side of her abdomen.
The other interesting thing noted was the change in immunochemistry of the metastatic lesion. The primary tumour was estrogen and progesterone negative, and Her-2 positive. The metastatic nodule however was noted to be estrogen and progesterone positive. Niikura et al had reported that the incidence of discordance for ER, PR and HER2 between primary and metastatic tumours was 18.4%, 40.3% and 13.6% respectively [5]. As there are no clear treatment guidelines for such metastatic lesions, this change in immunohistochemistry provided us the option of putting the patient on hormonal therapy in the hope that it would control the disease. Treatment has to be individualized, based on the prognosis of the patient [6].
Due to its relatively rare occurrence, there is currently no consensus on the standard treatment for skeletal muscle metastases, and further studies are needed to determine the prognosis, and proper diagnostic and therapeutic treatment [7].
This patient will continue to be followed up closely with intermittent scans. However, her long-term prognosis is still undetermined.









