Abstract
Introduction: Polynucleotides (PNs) counteract menopause- and non-menopause-related atrophy. After enzymatic cleavage, hydrated PNs progressively release small nitrogen precursors (nucleosides, nucleotides, nitrogen bases) and show persistent moisturizing and viscoelastic effects.
Methods: Interventional phase: intra-vaginal (one per day) and vulvar (one or two per day) applications of a gynecological PNs-based cream as CE0373 medical device for up to four weeks to evaluate the persisting efficacy and safety of the PNs device on vulvovaginal atrophy (VVA) symptoms.
Results: All investigated VVA symptoms showed highly significant improvements, with more than 60% of women reporting symptom relief within two weeks of cream application. Improvements were most solid for vaginal burning (-72.5%), vaginal dryness (-71.4%), and dyspareunia (-71.0%), although benefits were still higher than 60% for vaginal itching and vaginal pain.
Conclusions: The indirect support of fibroblast vitality to help control menopause- and non-menopause-related atrophy and symptoms was the innovative polynucleotide-centred rationale that steered the development of the PNs-based formulation. Easy application and high compliance are further benefits of the cream device.
Keywords
Dyspareunia, PNs-based cream, polynucleotides, vulvovaginal atrophy, vulvovaginal itch
Introduction
According to international surveys, more than half of women experience vulvovaginal atrophy (VVA) symptoms within 4-5 years after menopause, with up to two-thirds of symptomatic women reporting moderate-to-severe symptoms [1]. Declining postmenopausal serum estrogen concentrations lead to changes in the vulvovaginal and vesicourethral areas, summarized as genitourinary syndrome of menopause (GSM): vaginal dryness, increased discharges, vulvovaginal pain, bleeding, burning, irritation, and itching. GSM sexual symptoms include decreased vaginal lubrication during sexual activity, dyspareunia, and decreased arousal, orgasm, and sexual desire [2,3].
Besides itching, irritation and other symptoms, the GSM emotional impact is severe, with half of Southern and Northern European women fearing pain and avoiding intimacy [4]. The role of atrophy and lesions of the vulva, namely the most easily accessible genital area, seems paramount if not exclusive. Recently, in a sample of postmenopausal women with dyspareunia screened for a trial with topical estrogens, swab touches over the vulvar vestibule evoked high median pain scores, while median vaginal mucosal pain was zero [5].
Whichever the cause or predisposing factor, including non-hormonal events such as trauma at delivery, recurrent infections, and surgical procedures, the reduction of subepithelial collagen and elastin, the loss of elasticity and the flattening of vaginal rugae are some of the hallmarks of the accelerated vulvovaginal ageing after menopause [6].
Many women with VVA symptoms resort to over-the-counter products such as vaginal lubricants and moisturizers, which international guidelines indicate as the first line of therapy in VVA treatment due to the lack of significant contraindications and side effects. Vaginal lubricants are used alone, especially when vaginal estrogen preparations are not accepted or contraindicated, or they may be combined with hormonal therapies.
The gynecological cream under evaluation contains polynucleotides (PNs) of biological origin, isostearyl lactate and glycerin and has long been commercially available as a CE0373 medical device. After enzymatic cleavage in tissues, natural-origin hydrophilic PNs from trout gonads progressively release small nitrogen precursors (nucleosides, nucleotides, nitrogen bases) and show persistent moisturizing and viscoelastic effects [7]. Thanks to their characteristics, PNs have been widely used for many years in various indications and districts in dermatology and plastic surgery to improve hydration, turgidity, and skin elasticity [8] and promote the healing of chronic wounds and ulcers [9]. Injectables medical devices combining PNs, and hyaluronic acid have been beneficial in treating symptoms related to vulvovaginal atrophy [10-11]. The emollient glycerin ingredient usefully softens and moisturizes vaginal mucosa and decreases itching; the formulation pH, which is like the vulvar skin pH, is a prerequisite for safe perineal and vulvar application. After vaginal application, the lactic acid released by local esterases helps maintain local pH around the physiological slightly acidic vaginal pH of 4.0-4.5 [12].
The topical vulvovaginal application of the PNs cream effectively counteracts vaginal dryness and other VVA symptoms. The PNs-based device may also be helpful in all conditions of menopause- and non-menopause-related vulvovaginal atrophy, poor lubrication, disrupted local pH, and support of self-repair of vaginal mucosa and vulvovaginal lesions [13].
The paper reports the outcomes of a real-world life study centered on a questionnaire distributed to patients using the gynecological PNs-based cream. Besides a standard assessment of VVA symptom relief, the anonymous paper questionnaire to survey the patients of a panel of obstetrics and gynecology specialists was the distinctive feature of the study. The investigation was the first of a long-term program to monitor the clinical performance and safety of the PNs device. Confirming the profile of known side effects and contraindications and identifying unknown side effects and emergent risks were other purposes of the study and the long-term monitoring program.
Materials and Methods
Prospective study cohort: adult women (≥18 years old) with VVA symptoms of menopausal and non-menopausal origin without known hypersensitivity or previous allergic reactions to any ingredient of the study formulation. Investigational Product: Class III CE0373 medical device, non-sterile cream for topical use packaged in 30-mL aluminium tubes with six vaginal applicators containing fish-extracted PNs, isostearyl lactate and glycerin. The presence of the two latter ingredients strengthens the moisturizing and lubricating activity of the product on the cervicovaginal mucosa, vulva, and perineum; they also cooperate to promote the physiological repair processes.
The interventional phase preliminary to the final assessment and survey called for the intra-vaginal (one per day) and vulvar (one or two per day) cream applications for up to four weeks to evaluate the persisting efficacy and safety of the PNs-based cream medical device on VVA symptoms.
Persisting efficacy on VVA symptoms
Self-assessment by the patient, at baseline and after the last cream application, with a battery of Likert-like clinical severity scale for the main VVA symptoms: vaginal dryness, vulvovaginal irritation and itching, vulvovaginal soreness, dyspareunia. Scale severity scores: 0 = absent, 1 = mild, 2 = moderate, 3 = strong, 4 = very strong [14].
The reason to use five-point Likert scales instead of 10-point scales was that outcomes assessed on five-point scales have unimodal and symmetric distributions while 10-point scales have highly skewed J- and U-shaped distributions. Outcomes assessed on five-point scales also have lower means and floor and ceiling effects. At the same time, regression analysis shows that such scales account for a significant fraction of total variance in both floor and ceiling effects and minimize the contribution of unaccounted factors [15].
Besides anonymous demographics, the survey questionnaire assessed the women’s menopausal status and their reasons for self-administering the PNs-based vulvovaginal cream.
Safety assessment
Based on spontaneous reporting by cohort subjects over the whole study period. The questionnaire included closed and open questions to identify known side effects, describe their clinical presentation and severity (mild, moderate, severe, duration), and identify any previously unknown adverse event.
Statistics
The sample size was estimated with the G*Power statistical program version 3.14 based on the worst-case hypothesis and considering two effect sizes [16]. The sample size calculation was performed considering the total symptom score and assuming a conservative 60%-improvement after topical application of the PNs-based device due to the lack of specific literature. Statistical analysis compared the mean scores for overall and individual VVA symptoms before and after treatment with the PNs-based device (paired two-sample Student’s t-test, StatPlus, statistical analysis program release v7 [17]).
The treatment effects were analyzed with the paired-sample Student’s t-test for within-patient variations (also called the dependent sample t-test) to assess if the mean difference between two sets of observations is zero [18]. Calculations for time-to-effect included testing for 3, 7, 14, 28 or more days. All statistical tests were two-sided with 95% confidence intervals (5% significance level).
Under the illustrated assumptions and considering the total average improvement score as the focus of the estimated sample size, the power to detect a significant difference would have been greater than 0.954 with a treatment cohort of at least 39 VVA women.
Results
Prospective treatment cohort
Fifteen investigators working in Italian private clinics and already prescribing the PNs-based cream medical device under study enrolled 61 women in the treatment cohort with VVA of menopausal or non-menopausal origin. Table 1 illustrates the women’s demographics, menopausal condition, and use of estrogens. For the overall cohort, including non-menopausal women, the mean period with VVA symptoms was 35.2 ± 43.04 months (range, two months to 20.0 years), with a little more than one-third of women experiencing VVA symptoms for 4 to 5 years (Figure 1), 47 women (77.0%) complaining that the VVA symptoms negatively affected their sexual activities, and 25 women (41.0%) reporting a negative impact on sexual drive.
Age |
Cohort mean ± SEM |
53.7 (10.95) |
Range (years) |
30-79 |
Menopause |
Yes |
38 (62.3%) |
No |
23 (37.7%) |
Oral estrogens |
Yes |
13 (21.3%) |
No |
48 (78.7%) |
Topical estrogens |
Yes |
6 (9.8%) |
No |
55 (90.2%) |
Table 1. Cohort women’s demographics, menopausal condition, and use of estrogens. SEM, standard error of the mean
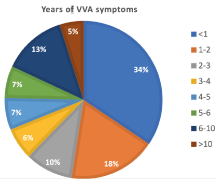
Figure 1. Years with VVA symptoms in the overall cohort of menopausal and non-menopausal women
Efficacy
Table 2 illustrates the reasons reported by the women for adopting the PNs-based cream treatment. Twenty-two women applied the PNs-based cream device once daily (36.1%), 24 twice daily (39.3%) and 15 more than twice daily (24.6%). Figure 2 shows the percentage change of the overall VVA symptom score at the study end compared with baseline; Figure 3 analytically illustrates the score changes for each VVA symptom. More in detail, Figure 4 illustrates how the clinical severity of vaginal dryness, the most frequently reported VVA symptom, decreased from baseline in each cohort’s woman.
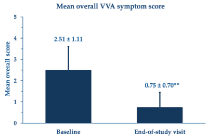
Figure 2. Improvement of the mean overall VVA symptom score (± standard errors of the mean) over the four-week study period. **p <0.0001 vs. baseline
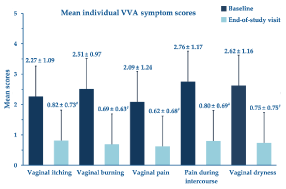
Figure 3. Mean score changes for each VVA symptom (± standard errors of the means) over the four-week study period; #p <0.0001 vs. baseline
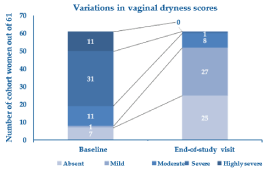
Figure 4. Subgroups of the women who reported vaginal dryness of variable clinical severity (scores: 0 to 4) at baseline and the end-of-treatment assessment visit
The control of VVA symptoms was rapid, with more than 60% of the cohort women reporting symptom relief within two weeks (Figure 5).
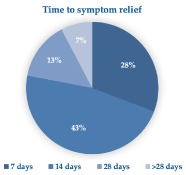
Figure 5. Time (days) elapsed before the cohort women reported symptom relief
| |
N |
% |
Vaginal/genital itching |
28 |
45.9 |
Vaginal burning |
23 |
37.7 |
Vaginal pain |
8 |
13.1 |
Pain/discomfort only during intercourse |
28 |
45.9 |
Vaginal dryness |
45 |
73.8 |
Other reasons |
- |
- |
Table 2. The cohort women’s reasons for adopting the proposed PNs-based treatment (assessed at baseline, more than one answer allowed in the questionnaire)
Safety
Treatment with PNs-based cream was well tolerated, with a few transitory and mild local adverse effects at the administration sites: mild burning in six women (9.8%), itching in three (4.9 %), and discomfort in eight (13.1%). All adverse effects resolved spontaneously in a few hours without treatment, and there were no unexpected side events.
Conclusion
Unlike the somewhat artificial setting of randomized controlled trials, real-world studies may provide insights, clinical data, and outcomes in conditions much like every day clinical practice [19]. Real-world studies are most useful in vulvovaginal atrophy and other menopausal and non-menopausal disorders due to their clinical polymorphism, high incidence, and psychological impact. Contrary to vasomotor symptoms, which tend to be milder over time, GSM symptoms have possibly a more significant impact on the women’s quality of life, confidence, self-esteem, and intimacy with their partners: they rarely resolve spontaneously and may deteriorate without treatment [2,3].
The PNs-based cream medical device under investigation might help counteract the signs of vulvovaginal atrophy and ageing. In the real-world study, PNs confirmed the efficacy and lack of clinically relevant side effects of the cream formulation after once-daily intra-vaginal and once or twice daily vulvar applications claimed in the Information for Use leaflet. The cohort of enrolled VVA women was pretty numerous, compensating for the lack of a control group—in fact, more numerous than the size estimated to reduce the risk of failing to detect a significant efficacy difference to almost zero.
Most patients suffered from the surveyed symptoms, even with different intensities, confirming the survey’s sound design and supporting the initial question’s rationale—why choosing the topical vaginal cream. All investigated VVA symptoms showed highly significant improvements, with more than 60% of women reporting symptom relief within the first two weeks of cream application; improvements were most remarkable for vaginal burning (-72.5%), vaginal dryness (-71.4%), and dyspareunia (-71.0%), although they were higher than 60% also for vaginal itching and vaginal pain. The clinically meaningful relief of VVA symptoms was not associated with more than some occasional undefined discomfort and mild burning and itching in a few cohort women.
In conclusion, the study demonstrated that the PNs-HTP®-based vaginal cream relieves the symptoms of vaginal dryness. Therefore, the cream is not only a valid alternative for patients unwilling or unable to consider estrogen therapy, but it is also suitable for general use in treating vaginal dryness symptoms, whatever the cause.
Conflicts of interest
The authors were formerly R&D consultants to Mastelli S.r.l., Sanremo, Italy (PNs producer). They certify that, at present, they have no conflict of interest with any financial or commercial organization concerning the manuscript’s content.
Acknowledgements
The authors wish to thank Dr Mauro Raichi for helping with analyses and discussion of outcomes and medical writing.
References
- Krychman M, Graham S, Bernick B, Mirkin S, Kingsberg SA (2017) The women’s EMPOWER survey: women’s knowledge and awareness of treatment options for vulvar and vaginal atrophy remains inadequate. J Sex Med 14: 425-433. [Crossref]
- Palacios S (2009) Managing urogenital atrophy. Maturitas 63: 315-318. [Crossref]
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Dolores Juliá M et al. (2005) Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas 52: S46-52. [Crossref]
- Nappi RE, Mattsson L-Å, Lachowsky M, Maamari R, Giraldi A, et al (2013) The CLOSER survey: impact of postmenopausal vaginal discomfort on relationships between women and their partners in Northern and Southern Europe. Maturitas 75: 373-379. [Crossref]
- Goetsch MF, Garg B, Lillemon J, Clark AL (2022) Where does postmenopausal dyspareunia hurt? A cross-sectional report. Menopause 29: 646-653. [Crossref]
- Mac Bride MB, Rhodes DJ, Shuster LT (2010) Vulvovaginal atrophy. Mayo Clin Proc 85: 87-94. [Crossref]
- Colangelo MT, Govoni P, Belletti S, Squadrito F, Guizzardi S, et al (2021) Polynucleotide bio gel enhances tissue repair, matrix deposition and organisation. J Biol Regul Homeost Agents 35: 355-362. [Crossref]
- Cavallini M, Bartoletti E, Maioli L, Massirone A, Palmieri IP, et al. (2021) Consensus report on the use of PNs-HPT™ (polynucleotides highly purified technology) in aesthetic medicine. J Cosmet Dermatol 20: 922-928. [Crossref]
- Maioli L (2020) Polynucleotides and nucleotides for the acceleration and regulation of normal wound healing. Aesthetic Medicine 6: 48-52.
- Palmieri IP, Raichi M (2019) Biorevitalization of postmenopausal labia majora, the polynucleotide/hyaluronic acid option. Obstet Gynecol Rep 3: 1-5.
- Angelucci M, Frascani F, Franceschelli A, Lusi A, Garo ML, et al. (2022) Efficacy of intradermal hyaluronic acid plus polynucleotides in vulvovaginal atrophy: a pilot study. Climacteric 25: 1-7. [Crossref]
- Mårdh P-A, Novikova N, Niklasson O, Bekassy Z, Skude L (2003). Leukocyte esterase activity in vaginal fluid of pregnant and non-pregnant women with vaginitis/vaginosis and in controls. Infect Dis Obstet Gynecol 11: 19-26. [Crossref]
- Trojano G (2005) Review on polynucleotides and benign lesions of the portio, vagina, vulva and perineum. Il Punto in Ginecologia 3: 2-4.
- Ettinger B, Hait H, Reape, Kathleen Z, Shu H (2008) Measuring symptom relief in studies of vaginal and vulvar atrophy. Menopause 15: 885-889. [Crossref]
- Garratt AM, Helgeland J, Gulbrandsen P (2011) Five-point scales outperform 10-point scales in a randomised comparison of item scaling for the Patient Experiences Questionnaire. Journal of Clinical Epidemiology 64: 200-207.
- Faul F, Erdfelder E, Buchner A, Lang A-G (2009) Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 41: 1149-1160. [Crossref]
- https://www.analystsoft.com/en/products/statplus/#statfeatures.
- Mishra P, Singh U, Pandey CM, Mishra P, Pandey G (2019) Application of Student’s t-test, analysis of variance, and covariance. Ann Card Anaesth 22: 407-411. [Crossref]
- Miksad RA, Abernethy AP (2018) Harnessing the power of real‐world evidence (RWE): a checklist to ensure regulatory‐grade data quality. Clin Pharmacol Ther 103: 202-205. [Crossref]





