Abstract
Purpose: A simple model was designed based on the degree of reduction in prealbumin concentration and T-lymphocyte count in critically ill patients, to investigate whether a simple score model could be used to assess mortality in critically ill patients.
Methods: Stratification was performed according to prealbumin concentration and lymphocyte count reduction, The normal concentration of prealbumin was 170-420mg/L, ≥normal concentration were scored as 0 points; 110-170 mg/L was scored as 1 point; ≤110 mg/L was recorded as 2 points; T lymphocyte normal count is 955-2860 /ul, ≥normal count was recorded as 0 point, 955-455 /ul was recorded as 1 point; ≤455 /ul score was recorded as 2 points. Combinational scores were performed to explore the correlation between combined scores and 28-day mortality and APACHE II.
Results: There were 19 patients (8.05%) with a combined score of 0, 35 patients (14.83%) with a combined score of 1, 62 patients (26.27%) with a combined score of 2, 66 patients (27.97%) with a combined score of 3, and 54 patients (22.88%) with a combined score of 4. Patients with larger scores were older, had lower T lymphocyte count, lower red blood cell count, lower hemoglobin concentration, lower prealbumin concentration, lower albumin concentration, and higher C-reactive protein concentration. Patients with higher combined score had higher 28-day mortality, which was statistically significant (P < 0.05).
Conclusion: A simple combined score of prealbumin concentration and lymphocyte count can be used to predict mortality in critically ill patients.
Keywords
critically ill patients, prealbumin, lymphocytes,prognosis
Introduction
Patients in intensive care unit (ICU) often had a poor prognosis due to their complex and rapidly changing conditions. Data showed that the inpatient mortality of severe patients was 42.5% [1]. The current ICU patient scoring system includes APACHE II scoring, GCS scoring and SOFA scoring, and so on. APACHE II scoring is widely used and has good prognostic value.However, indicators reflecting nutritional and immune status of patients were not included. Nutrition and immunity were two important factors which affect the prognosis of patients. Therefore, we urgently need to find a simple scoring model in the early stage, so as to judge and implement intervention as early as possible and reduce the mortality of patients. Plasma protein level can reflect the nutritional status of protein and the severity of disease. Many biological markers had been used in clinical evaluation of nutritional status, but no one had been found to be completely satisfactory [2]. Albumin was not suitable for assessing acute changes in nutritional status due to its long half-life(15-19 days)but should perform better as an indicator of chronic malnutrition. Transferrin was dependent on iron status and can therefore be reduced in malnutrition, but iron deficiency, which may be associated with nutritional disorders, can increase its serum concentration. Therefore, this method was no longer recommended for nutritional assessment [3]. Insulin-like growth factor 1 levels were reduced in malnourished patients, and its effectiveness depended on its very short half-life. It was just 6 hours. Retinol conjugated protein provided exactly the same information as prealbumin, but its detection cost was higher, and it is more sensitive to renal disease[4]. In contrast, prealbumin analysis was easy to perform in the laboratory, and prealbumin described a patient at risk of developing malnutrition, not a patient who was already malnourished [5,6]. Prealbumin was a thyroid hormone transporter that was synthesized by the liver and catabolized in part by the kidneys. Prealbumin was a common indicator of laboratory nutritional status, and serum prealbumin concentration below 10 mg/dL was associated with malnutrition [7]. Studies had shown that the critical level of serum prealbumin at 123.43mg/ L is an independent risk factor for death [8].Compared with other serum proteins, prealbumin was less affected by liver disease, even though it was synthesized by the liver. Lymphocyte was an important immune cell in human body, and the difference in lymphocyte subpopulation level can reflect the immune state of the people, and the decrease of lymphocyte indicated the damage of immune system, which had diagnostic and prognostic value [9]. Studies have pointed out that the degree of initial lymphocyte reduction (especially T lymphocyte) in ICU patients with severe infection is related to the prognosis, and the lower the lymphocyte level is, the worse the prognosis is[10]. When the cut-off value is 453 /μl,CD3 +T lymphocyte count has the highest predictive value for the patients with severe pneumonia[11]. In addition, studies have shown that prealbumin has thymus activity and can enhance body immunity by promoting lymphocyte matured [12]. Clinically, it was often found that prealbumin concentration and lymphocyte count in critically ill patients were lower than those in general wards. This study aimed to design a simple score model based on the prealbumin concentration and T lymphocyte count reduction degree of critically ill patients. We chose the critical value of prealbumin as 110mg/l, the critical value of lymphocyte as 455/ul. So as to explore whether simple score can be used to evaluate the mortality of critically ill patients and provided an early judgment for the prognosis of critically ill patients clinically.
Methods
The general information
This study was a retrospective study, collecting the data of patients in the ICU of The First Affiliated Hospital of Zhengzhou University from September 2020 to September 2021. Inclusion criteria were: All patients over 18 years of age admitted to the ICU with an expected ICU stay of more than 72 hours. Exclusion criteria were: (1) Those over 18 years of age who are expected to stay in ICU for less than 72 hours; (2) those who refuse to be included in the study or have participated in similar studies; (3) complicated with organ malignant tumor; (4) persons with mental disorders; (5) pregnant women; (6) Any disease causing primary or acquired immune deficiency, such as HIV, active autoimmune disease, blood disease and so on, or had received chemotherapy within the past three months; (7) Incomplete follow-up data. Excluding the cases that lacking sufficient data, a total of 236 patients were included. This study was in accordance with the Helsinki Declaration and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Data collection
Collecting the results of the patient's first blood test within 24 hours of admission. Serum prealbumin levels were determined by immunoturbidimetry. Other general data were collected, including age, sex, T lymphocyte count, red blood cell count, white blood cell count, platelet count, hemoglobin, prealbumin, albumin, C-reactive protein, conjugated bilirubin, unconjugated bilirubin, total bilirubin, B-type brain natriuretic peptide, APACHEII, and so on.
Follow-up
The end point of follow-up was the 28-day mortality rate from the day of admission to the intensive care unit, based on course records and telephone consultations.
Grouping
Stratification was performed according to prealbumin concentration and lymphocyte count reduction, The normal concentration of prealbumin was 170-420mg/L, greater than or equal to normal concentration were scored as 0 points; 110-170 mg/L was scored as 1 point; less than or equal to 110 mg/L was recorded as 2 points; T lymphocyte normal count is 955-2860 /ul, greater than or equal to normal count was recorded as 0 point, 955-455 /ul was recorded as 1 point; less than or equal to 455 /ul score was recorded as 2 points. Combinational scores were performed to explore the correlation between combined scores and 28-day mortality and APACHE II.
Statistical analysis
For continuous variables, the computed data conforming to normal distribution were expressed as mean ± standard deviation (SD), and variance analysis was done for comparison among groups. For categorical variables, a chi-square test was used for comparison among groups. Kaplan-Meier survival analysis was computed to compare the cumulative survival rates of the five groups. All statistical analyses were performed using the SPSS software version 26.0 (SPSS, Chicago, IL, USA). A two-sided P value of 0.05 was adopted as statistically significant.
Results
There are a total of 236 patients were categorized. As shown in Table 1, there were 19 patients (8.05%) with 0, 35 patients (14.83%) with 1,62 patients (26.27%) with 2, 66 patients (27.9%) with 3, 54 patients (22.88%) with 4, The patient had the higher score, who had the older the age, the lower T lymphocyte count, red blood cell count and hemoglobin concentration, the lower the prealbumin concentration and albumin concentration, the higher the C-reactive protein concentration. Patients with a higher 28-day mortality rate had a higher combined score, which was statistically significant(P< 0.05). This suggests timely nutritional supplementation at the early stage of admission to the ICU, to prevent anemia and infection. And comorbidity do not affect the combined scores.
|
Combined score
|
|
|
0
|
1
|
2
|
3
|
4
|
P
|
|
|
19(8.05%)
|
35(14.83%)
|
62(26.27%)
|
66(27.97%)
|
54(22.88%)
|
|
Gender
|
|
|
male
|
14
|
24
|
40
|
42
|
35
|
0.93
|
|
female
|
5
|
11
|
22
|
24
|
19
|
|
Age
|
49.00 ± 12.671
|
50.23 ± 15.088
|
54.53 ± 15.041
|
60.73 ± 13.968
|
59.22 ± 17.101
|
<0.05
|
|
Cell
|
|
|
lymphocyte
|
1409.34 ± 386.23
|
855.45 ±
326.99
|
685.52 ±
607.22
|
457.76 ± 214.64
|
280.70 ± 137.89
|
<0.05
|
|
erythrocyte
|
3.99 ± 0.87
|
3.68 ± 0.86
|
3.72 ± 0.83
|
3.43 ± 0.78
|
3.37 ± 0.71
|
<0.05
|
|
hemoglobin
|
120.62 ± 26.17
|
110.20 ± 26.92
|
111.80 ± 26.61
|
103.38 ± 24.32
|
101.56 ± 23.29
|
<0.05
|
|
thrombocyte
|
221.58 ±
80.04
|
228.00 ±
109.28
|
192.66 ±136.52
|
202.88 ± 130.61
|
151.09 ± 112.12
|
<0.05
|
|
leukocyte
|
12.99 ± 4.74
|
12.89 ± 5.04
|
13.69 ± 7.53
|
13.05 ± 6.42
|
12.95 ± 7.41
|
0.97
|
|
neutrophil
|
14.93 ± 19.04
|
11.07 ± 4.70
|
11.64 ± 6.48
|
11.44 ± 6.28
|
11.60 ± 7.11
|
0.51
|
|
Protein
|
|
|
prealbumin
|
227.47 ± 39.86
|
179.74 ± 44.07
|
150.35 ± 68.13
|
106.17 ± 37.82
|
72.72 ± 21.39
|
<0.05
|
|
albumin
|
39.17 ± 4.03
|
33.82 ± 5.73
|
32.66 ± 7.06
|
32.15 ± 6.07
|
29.88 ± 5.55
|
<0.05
|
|
CRP
|
38.05 ±
53.24
|
43.38 ±
44.05
|
72.49 ±
67.17
|
110.77 ± 107.89
|
130.27 ±
85.90
|
<0.05
|
|
Other
|
|
|
ASL
|
140.42 ±
323.64
|
44.89 ± 26.82
|
345.24 ±
192.04
|
95.94 ±
187.31
|
123.69 ±
304.83
|
0.58
|
|
ALT
|
92.21 ± 239.96
|
35.60 ± 34.03
|
138.23 ± 429.56
|
82.83 ± 135.27
|
78.00 ± 182.53
|
0.42
|
|
DBIL
|
5.32 ± 3.54
|
6.56 ± 4.22
|
15.00 ± 29.46
|
24.60 ± 52.11
|
29.39 ± 51.55
|
<0.05
|
|
IBIL
|
7.06 ± 3.52
|
6.79 ± 3.47
|
9.92 ± 9.30
|
10.90 ± 12.07
|
14.40 ± 20.99
|
0.058
|
|
TBIL
|
12.38 ± 6.67
|
13.35 ± 6.85
|
25.62 ± 36.99
|
35.50 ± 62.79
|
43.79 ± 68.85
|
<0.05
|
|
NT-BNP
|
1472.20 ±
3103.95
|
1291.10 ±
2784.15
|
2250.89 ±
5833.26
|
2965.93 ± 5129.94
|
7110.40 ± 2427.52
|
0.144
|
|
Co-disease
|
|
|
hypertension
|
|
|
yes
|
9
|
9
|
17
|
31
|
19
|
0.08
|
|
no
|
10
|
26
|
45
|
35
|
34
|
|
diabetes
|
|
|
yes
|
2
|
4
|
8
|
16
|
12
|
0.26
|
|
no
|
17
|
31
|
54
|
50
|
42
|
|
hepatopathy
|
|
|
yes
|
0
|
0
|
1
|
1
|
4
|
0.13
|
|
no
|
19
|
35
|
61
|
65
|
50
|
|
nephropathy
|
|
|
yes
|
0
|
1
|
0
|
1
|
1
|
0.76
|
|
no
|
19
|
34
|
62
|
65
|
53
|
|
CHD
|
|
|
yes
|
1
|
2
|
5
|
4
|
5
|
0.95
|
|
no
|
18
|
32
|
57
|
62
|
49
|
|
APACHEII
|
14.63 ± 7.63
|
16.11 ± 8.08
|
17.13 ± 8.67
|
18.27 ± 7.77
|
18.27 ± 7.77
|
0.39
|
|
Time
|
19.05 ± 17.46
|
17.57 ± 18.08
|
17.79 ± 16.80
|
14.24 ± 12.82
|
14.85 ± 12.95
|
0.53
|
*CHD means coronary heart disease, Co-disease means comorbidity.
Table 1. Baseline demographic, clinical, and laboratory values grouped by combinational score
|
Co-score
|
n
|
Death
|
Survival
|
Survival rate
|
|
0
|
19
|
4
|
15
|
78.94%
|
|
1
|
35
|
9
|
26
|
74.29%
|
|
2
|
62
|
24
|
38
|
61.29%
|
|
3
|
66
|
26
|
40
|
60.60%
|
|
4
|
54
|
23
|
31
|
57.40%
|
|
n
|
236
|
86
|
150
|
63.60%
|
Table 2. Comparison of survival rates in different groups(P=0.042)
As shown in Table 2, the survival rates of patients in 0 score group was 78.94%, the survival rates of patients in 1 score groups was 74.29%, the survival rates of patients in 2 score groups was 61.29%, the survival rates of patients in 3 score groups was 60.60% and the survival rates of patients in 4 score groups was 57.40%. It was statistically significant (P < 0.05). This suggests that the patients who had the higher score, who had the lower the survival rate. The combined score was inversely proportional to survival rate.
The following studies were computed to see differences in combined scores grouped by mortality and age. As shown in Table 3, the APACHEII of the death group was 20.85±8.467, and the APACHEII of the survival group was 15.29±7.412, with statistically significant differences (P < 0.05); The combined score of the death group was 2.64±1.126, and that of the survival group was 2.31±1.253, which was statistically significant (P < 0.05). Neither APACHEII or combined scoresthe scores of the death group were higher than the survival group. Stratified by age 18-29, 30-49, 50-69, and 70-89, as shown in Table 4, the combinational score of 18-29 was 2.21±1.251,the combinational score of 30-59 was 2.02±1.318,the combinational score of 50-69 was 2.45±1.182,the combinational score of 60-89 was 2.94±0.998,which was statistically significant(P< 0.05). This suggests older patients had higher combined scores and lower survival rates. The older patients were, the more attention should be paid to the occurrence of malnutrition and the decrease of immunity in clinical work.
|
|
n
|
Co-score
|
APACHEII
|
|
Death
|
86
|
2.64 ± 1.126
|
20.85 ± 8.467
|
|
Survival
|
150
|
2.31 ± 1.253
|
15.29 ± 7.412
|
|
|
|
P=0.043
|
p=0
|
Table 3. Comparison of combined score and APACHE II in the death group and the survival group (P=0.042, P=0)
|
Age
|
n
|
M ± SD
|
|
18-29
|
14
|
2.21 ± 1.251
|
|
30-49
|
58
|
2.02 ± 1.318
|
|
50-69
|
116
|
2.45 ± 1.182
|
|
70-89
|
48
|
2.94 ± 0.998
|
Table 4. combined score between different age groups (P=0.002)
As shown in Figure 1, when the observation time was 28 days, the cumulative survival rate of score 0-4 showed an overall decreasing trend, and the cumulative survival rate of score summation 3 and score summation 4 were relatively similar. The median survival time of score 0-4 was respectively 22.938 days, 21.463 days, 19.328days, 18.612 days and 18.472days. The median survival time was obviously different between groups even the significance level was 0.431.
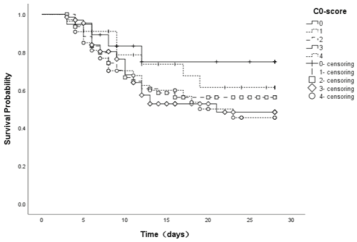
Figure 1. 28-day survival curves for groups 0-4
Discussion
Serum prealbumin is a sensitive and stable indicator of nutritional status. Serum prealbumin level has been proved to be correlated with the prognosis of patients with various diseases [13-16]. The results of this study showed that with the decrease of prealbumin concentration, the mortality of severe patients gradually increased, consistently with previous studies. Wen ji Wang and his partners showed that prealbumin predicted 90-day mortality of patients with acute kidney injury after controlling for other nutritional and inflammatory markers. Serum prealbumin levels decreased compared with patients with stable prealbumin levels; the prealbumin of patients decreased at 4 mg/dL,had a 79% increased risk of death [17]. Ju-dong Li and his partners showed that pre-operative prealbumin levels can be used to predict long-term prognosis in hepatectomy patients with Hepatocellular Carcinoma. Overall survival and progression-free survival were significantly reduced in patients with low prealbumin compared with normal prealbumin group[18]. Angelo and his partners showed that lower serum prealbumin concentration were significantly associated with severity and mortality of novel Coronavirus 2019 (COVID-19) [19]. However, the exact mechanism of the lower serum prealbumin concentrations observed in high-risk COVID-19 patients was unknown and may be related to common excessive inflammation and cytokine release [20,21]. Due to the complex and rapidly changing conditions of patients in intensive care, low prealbumin levels at admission may be associated with poor prognosis of patients at later stage. Detection of prealbumin levels at admission may facilitate appropriate risk assessment of patients at early stage, leading to better treatment measures.
Lymphocytes are the core of the body's immune response, and the most clinically detected are T cell subsets, such as CD3+, CD4+, CD8+, CD4+/CD8+, which generally reflect the body's current immune function, state and balance level, and are of great significance for assisting in the diagnosis of some diseases, analyzing their pathogenesis, observing efficacy and prognosis. Studies have shown that the SOFA score is equal to 3 as the critical value divided into severe and critical groups, the number of CD3+, CD4+ cells, CD4+% and CD4+/CD8+ ratio in critical group were significantly lower than those in severe group. Early test of peripheral blood T lymphocyte number in ICU patients has a good clinical significance for the early diagnosis, evaluation and guidance of clinical treatment of ICU patients[22]. Novel coronavirus research is a hot spot in recent years. Studies had shown that hematologic changes were common, including lymphocytopenia, and the standardized changes of T cell subsets are the largest. The T lymphocytes played an important role in clearing virus-infected cells, and the cell counts were valuable in predicting disease severity and clinical outcome [23-25]. Studies had shown that,CD3+ T cells counts and time when the nucleic acid turned negative were independent risk factors for in-hospital death of COVID-19 patients[26]. A sharp decrease in the total number of lymphocytes means that coronaviruses can consume many immune cells and inhibit cellular immune function [27]. These studies suggest that the injury of T lymphocytes may be an important factor leading to the deterioration of patients' disease. Lymphocyte count was not only related to the severity of the disease, but also to the speed and degree of improvement. Therefore, early use of immunomodulators can improve the status of infection in critically ill patients. Detection of lymphocyte count on admission was very important for predicting prognosis of patients in intensive care.
A combined scoring system based on serum prealbumin concentration and lymphocyte count (CO-PAL) score of 2 can accurately predict the outcome of patients undergoing gastrectomy for stage II/III gastric cancer. Lymphocyte count less than 1.5×109/L was counted as 1 score, prealbumin concentration less than 180 mg/ L was counted as 1 score, the overall survival rates at 1, 3 and 5 years in Co-PAL score 0 group were 93.6%, 69.1% and 54.8%, respectively, which were significantly higher than those in Co-PAL score 1 group (90.8%, 59.7% and 43.7%, P=0.005), and co-PAL score 2 group (84.2%, 51.3%, 36.2%, P<0.001[28].
Although some studies have confirmed the effect of immunological and nutritional status on the prognosis of critically ill patients. But clinical studies of the combination of the two are rare. In this study, patients were stratified according to the decreased degree of prealbumin concentration and lymphocyte count, and a total of 5 groups of patients with a combined score of 0-4. The result showed that the higher score group, the worse the prognosis, which was statistically significant (P < 0.05). The patient had the higher score, who had the older the age, the lower T lymphocyte count, red blood cell count and hemoglobin concentration, the lower the prealbumin concentration and albumin concentration, the higher the C-reactive protein concentration. Patients with a higher 28-day mortality rate had a higher combined score, which was statistically significant (P< 0.05). With the increase of the combined score, the overall survival rate of patients showed a decreasing trend. The combined score of the death group was 2.64±1.126, and that of the survival group was 2.31±1.253, the difference was statistically significant (P < 0.05).
This suggests that the reduction of prealbumin and lymphocyte count had clinical significance. Nutrition should be given early to patients be admitted in the ICU, lymphocyte subsets need to be tested. APACHE II was used for the initial score of severe patients within 24 hours. The higher the score, the worse the prognosis was. Simple scoring model can be used to judge critically ill patients at the early stage (< 24 hours), when they were admitted in ICU, to take active intervention measures timely, which may improve the prognosis of patients and reduce their mortality.
However, in this study, the number of stratified groups were smallIf we increase the number of stratified groupsthe obvious difference in scores between groups may be seen intuitively. In addition, some patients were transferred to the hospital for treatment, and the prealbumin concentration and lymphocyte count were not measured in the early admission period of critically ill patients, which may affect the results to a certain extent. This is a retrospective study, and a large-scale prospective study is needed in the future. The influence of changes in prealbumin concentration and lymphocyte count on prognosis of patients can be further explored.
Conclusions
In conclusion, a simple combinational score based on prealbumin concentration and lymphocyte count is a good predictor of mortality. Measurement and calculation in the early stage of admission to the ICU, and active intervention can improve the prognosis of patients and reduce the mortality rate. In recent years, nutritional support has received extensive attention in the industry in the prevention and treatment of severe diseases. Studies have shown that adding immunomodulatory components into traditional enteral nutrition can stimulate the immune response, improve the immune defense function, and then reduce the mortality of severe disease. Therefore, by measuring the prealbumin concentration and lymphocyte count of critically ill patients, adopting immune-type enteral nutrition in an early and timely stage, it can effectively improve the prognosis and reduce the mortality of the disease [29,30].
References
- Lv RH, Guan W (2015) Clinical study on risk factors of mortality in ICU patients. Gansu medicine 2015: 92-94.
- de Vries J, Antoine JM, Burzykowski T, Chiodini A, Gibney M, et al. (2013) Markers for nutrition studies: review of criteria for the evaluation of markers. European journal of nutrition. Eur J Nutr 52: 1685-1699. [Crossref]
- Zhao HQ, Wu H, Meng R, Du S, Tao SJ (2015) Distribution of serum transferrin, and its associations with metabolic disorders among Chinese: A nation-wide, health and nutrition survey. Mol Nutr Food Res 59: 1535-1540. [Crossref]
- Xun C, Zhao Y, Wang W, Chen C (2018) Circulating RBP4 Increase and Its Diagnosis of Chronic Kidney Disease. Ann Clin lab Sci 48: 205-207. [Crossref]
- Shenkin A (2006) Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition? Clin Chem 52: 2177-2179. [Crossref]
- Caccialanza R, Palladini G, Klersy C, Cereda E, Bonardi C, et al. (2013) Serum prealbumin: an independent marker of short-term energy intake in the presence of multiple-organ disease involvement. Nutrition 29: 580-582. [Crossref]
- Keller U (2019) Nutritional Laboratory Markers in Malnutrition. J Clin Med 8. [Crossref]
- Tao XC, Lin JT, Liu GL, Li J, Li LJ, et al. (2015) The prognosis of respiratory critically ill patients and serum prealbumin concentration. J China-Japan Friendship Hospital 29: 158-160.
- Zhang X, Tan Y, Ling Y, Lu G, Liu F, et al. (2020) Viral and host factors related to the clinical outcome of COVID-19. Nature 583: 437-440. [Crossref]
- Hohlstein P, Gussen H, Bartneck M, Warzecha KT, Roderburg C, et al. (2019) Prognostic Relevance of Altered Lymphocyte Subpopulations in Critical Illness and Sepsis. J Clin Med 8. [Crossref]
- Wang F, Chen XX, Han ZH (2021) Proportional changes of T lymphocyte subsets in peripheral blood and their efficacy in predicting prog-nosis of patients in early stage of severe pneumonia. Shandong medicine 61: 32-36.
- Li LH, Tian G (2005) Research progress of serum prealbumin. International Journal of Laboratory Medicine.
- Kuszajewski ML, Clontz AS (2005) Prealbumin is best for nutritional monitoring. Nursing 35: 70-71. [Crossref]
- Caccialanza R, Palladini G, Klersy C, Cena H, Vagia C, et al. (2006) Nutritional status of outpatients with systemic immunoglobulin light-chain amyloidosis 1. Am J Clin Nutr 83: 350-354. [Crossref]
- Devakonda A, George L, Raoof S, Esan A, Saleh A, et al. (2008) Transthyretin as a marker to predict outcome in critically ill patients. Clin Biochem 41: 1126-1130. [Crossref]
- Rambod M, Kovesdy CP, Bross R, Kopple JD, Kalantar-Zadeh K (2008) Association of serum prealbumin and its changes over time with clinical outcomes and survival in patients receiving hemodialysis. Am Clin Nutr 88: 1485-1494. [Crossref]
- Wang W, Pan Y, Tang X, Hao G, Xie Y, et al. (2017) Serum prealbumin and its changes over time are associated with mortality in acute kidney injury. Sci Rep 7: 41493. [Crossref]
- Li JD, Xu XF, Han J, Wu H, Xing H, et al. (2019) Preoperative prealbumin level as an independent predictor of long-term prognosis after liver resection for hepatocellular carcinoma: a multi-institutional study. HPB (Oxford) 21: 157-166. [Crossref]
- Zinellu A, Mangoni AA (2021) Serum Prealbumin Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis. Front Med 8: 638529. [Crossref]
- Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I (2020) COVID-19 cytokine storm: The anger of inflammation. Cytokine 133: 155151. [Crossref]
- Liu F, Li L, Xu M, Wu J, Luo D, et al. (2020) Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 127: 104370. [Crossref]
- Zheng-you JIA (2016) Detection and clinical significance of immune typing of peripheral blood T lymphocyte subsets in severe patients. Guide of China Medicine 14: 8-10.
- Yang M, Li CK, Li K, Hon KL, Ng MH, et al. (2004) Hematological findings in SARS patients and possible mechanisms (review). Inter J Molecular Med 14: 311-315. [Crossref]
- He S, Zhou C, Lu D, Yang H, Xu H, et al. (2020) Relationship between chest CT manifestations and immune response in COVID-19 patients. Inter J Infect Dis 98: 125-129. [Crossref]
- Liu Y, Tan W, Chen H, Zhu Y, Wan L, et al. (2021) Dynamic changes in lymphocyte subsets and parallel cytokine levels in patients with severe and critical COVID-19. BMC Infect Dis 21: 79. [Crossref]
- Zhang P, Du W, Yang T, Zhao L, Xiong R, et al. (2021) Lymphocyte subsets as a predictor of severity and prognosis in COVID-19 patients. Int J Immunopathol Pharmacol 35: 20587384211048567. [Crossref]
- Liu WJ, Zhao M, Liu K, Xu K, Wong G, et al. (2017) T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral Res 137: 82-92. [Crossref]
- Shen Q, Liu W, Quan H, Pan S, Li S, et al. (2018) Prealbumin and lymphocyte-based prognostic score, a new tool for predicting long-term survival after curative resection of stage II/III gastric cancer. Br J Nutr 120: 1359-1369. [Crossref]
- Annetta MG, Pittiruti M, Vecchiarelli P, Silvestri D, Caricato A, et al. (2016) Immunonutrients in critically ill patients: an analysis of the most recent literature. Minerva anestesiologica 82: 320-331. [Crossref]
- Ali S, Roberts PR (2006) Nutrients with immune-modulating effects: what role should they play in the intensive care unit? Curr Opin Anaesthesiol 19: 132-139. [Crossref]

