Abstract
Introduction: Menopause and the physiological decrease in estrogen levels are often associated with vaginal atrophy-related symptoms - vaginal dryness, dyspareunia, burning, itching, decreased libido and overall worsened quality of life and interference with sexual activity. Polynucleotide (PNs)-based medical devices relieve menopause-related vaginal atrophy by increasing the hydration and lubrication of cervical and vaginal mucosae and favoring the physiological regenerative and reparative mechanisms.
Methods: Treatment: One vaginal ovule per day (PNs-based CE0373 medical device) for two to four weeks in a cohort of 54 post-menopausal women. Baseline and end-of-study evaluations: investigator-assessed, five-subscale Vaginal Health Index (VHI) assessing vaginal elasticity, the volume of vaginal secretions, intravaginal pH, and vaginal mucosal integrity and moisture. Vaginal atrophy cut-off score: <15 out of a total VHI score of 25.
Results: All investigated vulvovaginal atrophy signs showed highly significant improvements as judged by investigators. The cohort of women experienced the most solid improvements in vaginal hydration (+ 59.8%), vaginal secretions (+ 52.7%), and superficial petechiae (+ 51.6%); the improved deeper tissue elasticity was also remarkable (+ 50.9%).
Conclusions: In conclusion, PNs-based vaginal ovules are an efficient topical therapy for women suffering from vulvovaginal atrophy who have contraindications for estrogen therapy and suffering from vulvovaginal signs and symptoms affecting their quality of life. The study confirms that the PNs rationale retains its innovative potential. The study also confirms the medical device’s persisting high safety and women’s compliance to treatment.
Keywords
Dyspareunia, PNs-based ovules, polynucleotides, vulvovaginal atrophy, vulvovaginal itch
Introduction
During the peri- and post-menopausal hormonal changes, the estradiol levels precipitate from their pre-menopausal menstrual cycle peaks of 800 pg/mL to (adrenal androstenedione-derived) levels below 30 pg/mL. Itching, irritation, vaginal dryness, discomfort, and frank pain during sexual activity are atrophy-related symptoms of oestradiol fall [1]. The vaginal epithelia of post-menopausal women display flattened epithelial surfaces with features of keratinization and lack of papillae; concomitantly, the decreased saprophyte population of lactobacilli leads to increased intravaginal pH. The thinning of the vaginal epithelium increases susceptibility to trauma, resulting in bleeding, petechiae, and ulceration during sexual activity or gynecological maneuvers [1]. Thinning also exposes the underlying connective tissue, which is more vulnerable to inflammation or infection. The depleted collagen and elastin fibers of the vulvar skin and vulvovaginal lamina propria are responsible for lost elasticity and flattening of vulvar labia and vaginal rugae [1]. The chronic progressive atrophy of vulvar teguments is also the hallmark of atrophic and sclerosus genital lichen or vulvar kraurosis, with leucoplakia and petechiae, chronic inflammation in deeper tissues and involutional changes up to ulcerations, stenosis of the vaginal introitus and pre-cancerous lesions [2].
Many women with VVA use over-the-counter products such as vaginal lubricants and moisturizers. International guidelines consider those formulations as the first-line therapy of VVA because of the lack of significant contraindications and side effects. They can be used alone or combined with hormonal therapies as needed; vaginal lubricants and moisturizers are also recommended when vaginal estrogen preparations are not accepted or contraindicated.
A gynecological CE0373 medical device — vaginal ovules based on natural-origin, highly purified polynucleotides from trout gonads — may be helpful in all conditions of menopause and non-menopause-related vulvovaginal atrophy and support the self-repair of vulvar tears and other lesions [3-5]. PNs progressively release small nitrogen precursors (nucleosides, nucleotides, nitrogen bases) and shows persistent moisturizing and viscoelastic effects [6].
Glycerin, an emollient ingredient of the ovule formulation, usefully softens and moisturizes vaginal mucosa and decreases itching. Another ingredient, lactic acid, helps maintain the vaginal pH around its physiological acidic value, 4.0-4.5, and avoids the proliferation of microorganisms with pathogenic potential. Moreover, hydrophilic lactic acid is essential to preserve the correct vaginal moisture and prevent dryness and irritation [7].
The purpose of the illustrated prospective study was to confirm the efficacy and profile of known side effects and contraindications of the proprietary PNs-based vaginal ovules. The study was the first of a long-term program to monitor the experiences of gynecologists regarding the clinical efficacy and safety of the proprietary PNs-based vaginal ovules. Efficacy meant symptom relief and restoring a healthy vaginal environment. Efficacy is objectively evaluated in terms of improvement of vaginal signs and restoration of a healthy vaginal environment.
A second purpose was to identify any previously unknown side effects and emergent risks.
Materials and Methods
Prospective study cohort: adult women (≥18 years old) with vaginal atrophy-related signs of menopausal and non-menopausal origin and without known hypersensitivity or previous allergic reactions to ingredients of the study formulation.
Treatment: intravaginal insertion of one ovule per day for two to four weeks.
Primary validated assessment of efficacy and safety: a questionnaire designed explicitly with the topical vaginal application of PNs-based vaginal ovules in mind and distributed to a panel of obstetrics and gynecology specialists.
Assessment of efficacy
Relieving the VVA objective signs and restoring the vaginal environment to a healthy state is a leading therapeutic goal in VVA management. Therefore, the study’s primary efficacy endpoint was the improvement of vaginal signs from baseline, evaluated by the investigator in terms of change of a validated tool - the Vaginal Health Index (VHI) score.
First described in 1995, the Vaginal Health Index (VHI) is a five-point scale, a validated and investigator-assessed scoring tool that evaluates the vaginal elasticity and volume of vaginal secretions, intravaginal pH, and vaginal mucosal integrity and moisture (Table 1) [8]. VHI subscale scores: 1 = none, 2 = poor, 3 = fair, 4 = good, 5 = excellent. The total VHI score ranges between 5 and 25; total scores below 15 highlight vaginal atrophy [8].
Score |
Overall elasticity * |
Fluid secretion type and consistency |
pH |
Mucosal epithelium |
Moisture |
1 |
None |
None |
6.1 |
Petechiae noted before contact |
None; mucosa inflamed |
2 |
Poor |
Scant, thin yellow |
5.6−6.0 |
Bleeds with light contact |
None; mucosa not inflamed |
3 |
Fair |
Superficial, thin white |
5.1−5.5 |
Bleeds with scraping |
Minimal |
4 |
Good |
Moderate, thin white |
4.7−5.0 |
Not friable, thin mucosa |
Moderate |
5 |
Excellent |
Normal (white flocculent) |
≤4.6 |
Not friable, normal mucosa |
Normal |
Table 1. Vaginal Health Index (Bachmann et al.); *Lower scores correspond to progressively more severe urogenital atrophy [8]
The study’s primary endpoint was the VHI score change from baseline after one month of treatment and the last PNs vaginal ovule insertion.
The unimodal symmetric distributions of outcomes on five-point Likert-like assessment tools like the VHI subscales minimize the statistical obstacle of skewed J- and U-shaped distributions. Outcomes assessed on five-point scales also have lower means, floor, and ceiling effects. At the same time, regression analysis shows that these scales explain a significant fraction of the variation in both floor and ceiling effects while minimizing the contribution of unaccounted factors [9].
Safety assessment
Based on spontaneous reporting over the whole study period. The questionnaire included closed and open questions to identify known side effects, describe their clinical presentation, severity, and duration, and identify any previously unknown adverse event.
Statistics
Sample size estimation: based on total average improvement score under the assumption, based on a literature review of the efficacy of comparable products, of an improvement of 70% after the intravaginal PNs-based therapeutic cycle. Statistical software: G*Power version 3.14 [10]. Under the described assumptions, the power to detect a significant difference in an estimated treatment cohort of at least 39 women would have been greater than 0.954.
Statistical analysis of treatment effects: comparison of the mean scores for the total VHI score and subscores with the paired-sample t-Student test, also called dependent samples t‑test. Statistical analysis software: StatPlus, release v7 [11,12]). All statistical tests were two-sided with 95% confidence intervals (5% significance level).
Results
Treatment cohort
All experienced gynecologists and prescribers of PNs-based vaginal ovules, the nine participating clinical investigators enrolled 54 menopausal and non-menopausal women with vaginal atrophy. All women underwent treatment and completed the study fully compliant with the protocol with only a few transitory and mild local adverse effects and without spontaneous dropouts.
Table 2 illustrates the women’s demographics, menopausal or non-menopausal conditions, and use of estrogens. Only eight women (14.8%) reported the concomitant use of other treatments for their atrophy like flavonoid-based nutritional supplements, vaginal lubricants, or re-epithelizing ovules. For the overall cohort, including non-menopausal women, the mean period with symptoms was 43.9 ± 54.82 months (one month to 20.0 years), with a little less than one-third of women experiencing VVA symptoms for 1 to 3 years (Figure 1).
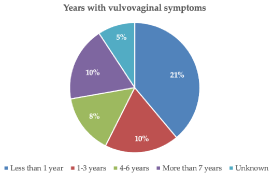
Figure 1. Years with atrophy symptoms in the overall women’s cohort (data available for 49 patients)
Age |
Cohort mean (SEM) |
52.2 (7.97) |
Range (years) |
37-71 |
Menopause |
Yes (%) |
33 (61.1) |
No (%) |
21 (38.9) |
Oral estrogens |
Yes (%) |
7 (13.0) |
No (%) |
47 (87.0) |
Topical estrogens |
Yes (%) |
8 (14.8) |
No (%) |
46 (85.2) |
Table 2. Cohort women’s demographics, menopausal condition, and use of estrogens. SEM, Standard Error of the Mean
The signs and symptoms of vaginal atrophy that mostly induced the investigators to prescribe the PNs-based intravaginal ovules were vaginal dryness (for 66.7% of women), vaginal itching and vaginal burning (both for 40.7% of women), vaginal pain (for 31.5% of women), often during intercourse (for 25.9% of women), and vaginal bleeding during intercourse (for 31.5% of women). All such severe symptoms occurred very commonly in combination. Vaginal surgery was another significant reason for prescribing the PNs-based ovules (for 20.4% of women). Table 3 details the evolution of scores in the subgroup of eleven women with vaginal atrophy developing as surgical sequelae.
Signs |
Baseline mean (SEM) |
End-of-treatment mean (SEM) |
Vaginal elasticity |
2.4 (0.77) |
3.2 (0.72) ** |
Vaginal secretions |
2.2 (1.11) |
3.9 (1.31) *** |
Vaginal pH |
2.3 (1.56) |
3.6 (1.34) *** |
Epithelial petechiae |
2.2 (1.11) |
4.2 (0.94) *** |
Vaginal hydration |
2.6 (1.44) |
3.7 (1.05) *** |
Table 3. Baseline and end-of-treatment scores in the eleven cohort women of the “post-surgery atrophy” subgroup. SEM, Standard Error of the Mean. **p <0.01, ***p <0.001.
Overall, 32 women (59.3%) complained that the baseline atrophic symptoms negatively affected their sexual life; 8 women (14.8%) also lamented the negative impact on their sexual desire.
Efficacy
At baseline, the mean total Vaginal Health Index score was 2.3 ± 1.09, meaning severe vulvovaginal atrophy. Although always with persisting atrophy signs and symptoms, Figure 2 shows the highly significant improvement in total VHI score at the study end compared with baseline.
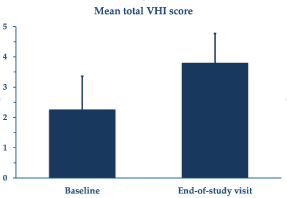
Figure 2. Mean total Vaginal Health Index score (± standard errors of the mean).
**p <0.0001 vs. baseline
Figure 3 analytically illustrates the improvements vs. baseline for each VHI subscale after treatment with the PNs-based ovules. Looking more in detail at the intravaginal pH outcomes (data available from 50 patients; Figure 4), the significant pH reduction in all women, including the eight women with a baseline pH of more than six, means that the whole treatment cohort experienced a generalized tendency towards the restoration of the physiologically acidic vaginal environment.
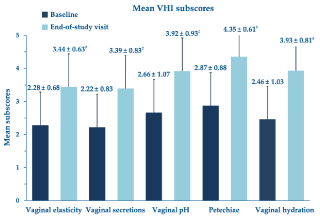
Figure 3. Mean VHI subscores (± standard errors of the mean); #p <0.0001 vs. baseline
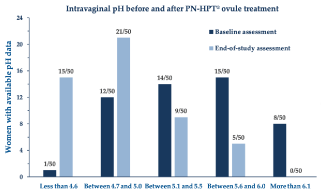
Figure 4. Intravaginal pH in the cohort’s women with available data at baseline (n=50) and the end-of-study treatment assessment visit
Finally, Figure 5 illustrates how the clinical severity of vaginal dryness, the most bothersome and frequently reported symptom of atrophy, decreased from baseline in each cohort’s woman. Out of 28 of the cohort women reporting severe or highly severe vaginal dryness before treatment, only one still suffered from severe dryness after a few weeks of self-administration of the PNs-based ovules.
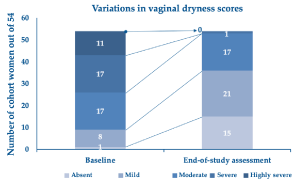
Figure 5. Cohort women who reported vaginal dryness of variable clinical severity (scores 1 to 5) at baseline and the end-of-treatment assessment visit
Safety
The repeated insertion of PNs-based ovules was well tolerated, with a few transitory and mild local adverse effects at the administration sites: mild burning in 22 women (40.7%), itching in 8 (14.8 %) and discomfort in 15 (27.8%). All adverse effects resolved spontaneously in a few hours without treatment, and there were no unexpected side events.
Conclusion
The purpose of real-world investigations is to provide reliable insights into conditions analogous to everyday clinical practice [13]. Within a real-world, long-term monitoring program, PNs in ovule formulation confirmed their efficacy and lack of bothersome side effects after once-daily intravaginal insertion in vulvovaginal atrophy and other menopausal and non-menopausal disorders or after surgery, as stated in the Information for Use leaflet.
The investigated PNs-based medical device was designed to support vaginal hydration, restore physiological vaginal pH, and counteract the signs of menopausal and non-menopausal vaginal atrophy.
The study, centered on the investigator-assessed Vaginal Health Index scale, had two main problems: compensating for the lack of a control group and the risk of failing to detect a significant difference in the severity of objective signs compared with baseline. The cohort of enrolled women was much larger than the size estimated to reduce to almost zero such risk under the conservative assumption of a 70% efficacy.
All investigated atrophy objective clinical signs showed highly significant improvements over a maximum treatment period of 4 weeks. Due to the strong PNs hydrating properties, it is not surprising that vaginal hydration posted the greatest improvement (+59.8% vs. baseline VHI subscore assessment). Vaginal secretions, petechiae and elasticity also showed solid improvements (+52.7%, +51.6% and +50.9%, respectively). The highly significant improvements also extended to all women of the “post-surgery atrophy” subgroup for all investigated parameters. PNs-based ovules are an effective option in all forms of vaginal atrophy, independently of cause. The clinically meaningful relief of VVA objective signs was not associated with more than some occasional undefined discomfort and mild burning and itching in a few cohort women.
In conclusion, improving VVA-related objective signs means that PNs-based vaginal ovules are an effective, safe, and well-tolerated non-hormonal therapeutic option in post-menopausal women.
Conflicts of interest
The authors were formerly R&D consultants to Mastelli S.r.l., Sanremo, Italy (PNs producer). They certify that, at present, they have no conflict of interest with any financial or commercial organization concerning the manuscript’s content.
Acknowledgements
The authors wish to thank Dr. Mauro Raichi for helping with analyses and discussion of outcomes and medical writing.
References
- Mac Bride MB, Rhodes DJ, Shuster LT (2010) Vulvovaginal atrophy. Mayo Clin Proc 85: 87-94. [Crossref]
- Cabrera AGC, Hernández MJM, Gómez CC (2016) Sclerosus and atrophic genital lichen or vulvar kraurosis. About a case. Medisur 14: 796-800.
- Trojano G (2005) Review on polynucleotides and benign lesions of the portio, vagina, vulva and perineum. Il Punto in Ginecologia 3: 2-4.
- Angelucci M, Frascani F, Franceschelli A, Lusi A, Garo ML. (2022) Efficacy of intradermal hyaluronic acid plus polynucleotides in vulvovaginal atrophy: a pilot study. Climacteric 25: 1-7. [Crossref]
- Maioli L (2020) Polynucleotides and nucleotides for the acceleration and regulation of normal wound healing. Aesthetic Medicine 6: 48-52.
- Colangelo MT, Govoni P, Belletti S, Squadrito F, Guizzardi S, et al (2021) Polynucleotide bio gel enhances tissue repair, matrix deposition and organization. J Biol Regul Homeost Agents 35: 355-362. [Crossref]
- CIR Cosmetic Ingredient Review. Safety Assessment of Alpha Hydroxy Acids as Used in Cosmetics. 2013.
- Bachmann G (1995) Urogenital ageing: an old problem newly recognized. Maturitas 22: S1-S5. [Crossref]
- Garratt AM, Helgeland J, Gulbrandsen P (2011) Five-point scales outperform 10-point scales in a randomised comparison of item scaling for the Patient Experiences Questionnaire. J Clin Epidemiol 64: 200-207. [Crossref]
- Faul F, Erdfelder E, Buchner A, Lang A-G (2009) Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods 41: 1149-1160.
- https://www.analystsoft.com/en/products/statplus/#statfeatures.
- Mishra P, Singh U, Pandey CM, Mishra P, Pandey G (2019) Application of Student’s t-test, analysis of variance, and covariance. Ann Card Anaesth 22: 407-411. [Crossref]
- Miksad RA, Abernethy AP (2018) Harnessing the power of real‐world evidence (RWE): a checklist to ensure regulatory‐grade data quality. Clin Pharmacol Ther 103: 202-205. [Crossref]





