Abstract
Olanzapine, a multi-acting receptor targeted antipsychotic drug, is effective for the prophylaxis as well as the rescue of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy or moderately emetogenic chemotherapy. Several clinical practice guidelines for antiemetic medication have recommended using olanzapine as the standard antiemetic therapy. However, little is known about cellular mechanisms underlying antiemetic action of olanzapine. Possible roles for ghrelin release and ghrelin receptor sensitization through blockade of 5-HT receptor subclasses in the antiemetic action of olanzapine are discussed.
Key words
Olanzapine, Chemotherapy-induced nausea and vomiting, 5-HT2B receptor, 5-HT2C receptor, Highly emetogenic chemotherapy, Moderately emetogenic chemotherapy
Chemotherapy-induced nausea and vomiting (CINV) was one of most distressing adverse events that patients complain during cancer chemotherapy [1]. However, the management of CINV has been greatly improved since the development of 5-HT3 receptor antagonists and neurokinin NK1 receptor antagonists [2-4]. Although the chemotherapy-induced vomiting is almost preventable by the use of the combination of three drugs, including 5-HT3 receptor antagonist, NK1 receptor antagonist and dexamethasone, the control of nausea during acute (day 1 of chemotherapy) and delayed periods (days 2-5) of chemotherapy remains unresolved [5,6].
Tan et al. [7] reported in a randomized controlled trial (RCT) evaluating the additional effect of olanzapine to the two-drug antiemetic medication consisting of azasetron and dexamethasone in patients receiving highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC) that the addition of olanzapine significantly improves the rates of no nausea (69.6% versus 30.4%, P<0.05) and no vomiting (78.6% versus 56.5%, P<0.05) during delayed period for HEC, and the rates of no nausea (83.1% versus 58.1%, P<0.05) and no vomiting (89.2% versus 75.8%, P<0.05) during delayed period for MEC. Navari et al. [8] also reported in a RCT comparing the effects of olanzapine and aprepitant for prevention of CINV in patients receiving cisplatin ≥70 mg/m2 or cyclophosphamide ≥500 mg/m2 and doxorubicin ≥50 mg/m2 that the rate of no nausea during delayed period is significantly higher in olanzapine group than in aprepitant group (69% versus 38%, P<0.01), although the rate of complete response (no emesis, no rescue) is similar between the two groups (77% versus 73%). The risk of nausea is particularly high in patients receiving anthracycline/cyclophosphamide combination (AC) chemotherapy for breast cancer. Iihara et al. [9] reported in 779 patients receiving first cycle of chemotherapy of any type of emetogenic risk that AC chemotherapy for breast cancer is at high risk for nausea (odds ratio: 4.955, 95% confidence interval: 1.863–13.18, P=0.001, as determined by a multivariate logistic regression analysis). Nawa-Nishigaki et al [10] showed that the addition of olanzapine (5mg orally per day for 5 days) to the three-drug antiemetic medication containing aprepitant, 5-HT3 receptor antagonist and dexamethasone remarkably improves the control of nausea, in which the rate of no nausea during delayed period is 89.5% and 50.7% in patients with and without additional olanzapine, respectively (P=0.005). Therefore, it is suggested that olanzapine is highly effective for the prophylaxis of chemotherapy-induced nausea.
Moreover, olanzapine is effective for the remedy of refractory or breakthrough CINV [11-13]. Navari et al. [11] showed in a double-blind, randomized phase III trial comparing the effects of olanzapine and metoclopramide on the breakthrough CINV in patients receiving HEC that olanzapine reduces the breakthrough CINV more potently than metoclopramide (68% versus 23%, P<0.01, for no nausea, 70% versus 31%, P<0.01, for no vomiting). The antiemetic effect of olanzapine has been confirmed by a number of studies [14-16] and their meta-analyses [17-21].
In 2014, the National Comprehensive Cancer Network (NCCN) recommended three-drug antiemetic medication containing olanzapine, palonosetron and dexamethasone as the standard antiemetic regimen for HEC and MEC. Thereafter, four-drug antiemetic medication consisting of olanzapine, 5-HT3 receptor antagonist, NK1 receptor antagonist, and dexamethasone is included in the NCCN guideline 2017 and the American Society of Clinical Oncology (ASCO) guideline 2017.
However, it is still uncertain how olanzapine fulfills its antiemetic action. Olanzapine is classified as the multi-acting receptor targeted (MARTA) antipsychotic drug that shows high affinity for dopamine D1, D2, D3 and D4 receptors, serotonin 5-HT2A, 5-HT2B, 5-HT2C, 5-HT6 and 5-HT7 receptors, histamine H1 receptor, muscarinic M1, M2, M3 and M4 receptors, and adrenergic a1A and a2C receptors [22]. The pKi values of olanzapine for various transmitter receptors are shown in Table 1. Olanzapine shows higher affinity for 5-HT2B and 5-HT2C receptors (pKi=8.41) than for dopamine D2 receptor (pKi=7.67). The ratios of Ki value for D2 receptors to those for 5-HT2B and 5-HT2C receptors are both 5.495, indicating that olanzapine has 5.495-fold higher affinity for 5-HT2B and 5-HT2C receptors than for D2 receptor. In contrast, the typical antipsychotic drug haloperidol shows negligible affinity for 5-HT2B or 5-HT2C receptor. Moreover, Bymaster et al. [23] reported that olanzapine potently inhibits the serotonin-stimulated accumulation of inositol 1,4,5-trisphosphate (IP3) in cell lines transfected with 5-HT2A or 5-HT2B receptors but weakly blocks serotonin-induced IP3 formation in cell lines transfected with 5-HT2C receptors. Taken together, physiologically relevant blockade of 5-HT2B and 5-HT2C receptors occurs after administration of olanzapine. Lord et al. [24] reported in mice that the hyperphagia induced by olanzapine is blocked by lorcaserin, a selective 5-HT2C receptor agonist [25], or diminished in 5-HT2C receptor knockout mice, thereby suggesting that olanzapine induces orexigenic action via blockade of 5-HT2C receptor.
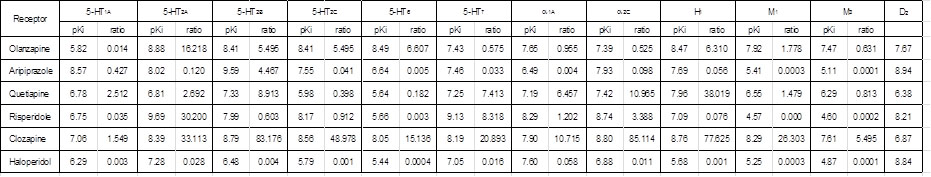
Table 1. Comparison of pKi for various neurotransmitter receptors among atypical and typical antipychotic drugs. Ratio of Ki for dopamine D2 receptor to Ki for each receptor as represented. pKi values were quoted from Shahid, et al. [22]
On the other hand, it has been demonstrated that cisplatin-induced decrease in appetite is mediated by stimulation of 5-HT2B and 5-HT2C receptors and subsequent reduction in the secretion of acylated ghrelin, an appetite-promoting gut peptide [26-28]. Takeda et al. [26] reported that administration of cisplatin (1-8mg/kg. i.p.) to rats decreases the plasma level of acylated ghrelin in a dose-dependent manner. Both the decrease in plasma acylated ghrelin and the reduction in food intake induced by cisplatin are reversed by the 5-HT2B receptor antagonist SB215505 and the 5-HT2C receptor antagonist SB242084. In addition, like cisplatin, m-chlorophenyl piperazine, a 5-HT2C receptor agonist, or BW723C86, a 5-HT2B receptor agonist, decreases plasma level of acylated ghrelin. These data suggest that serotonin liberated by cisplatin from enterochromaffin cells in guts stimulates both 5-HT2B and 5-HT2C receptors and subsequently inhibits the release of ghrelin, which causes a reduction in appetite.
These findings, taken together, suggest that olanzapine prevents CINV by stimulating the release of ghrelin via blockade of 5-HT2B and 5-HT2C receptors.
On the other hand, the modulation of ghrelin receptor signaling by 5-HT2C receptor has been demonstrated. Schellekens et al. [29] reported in human embryonic kidney (HEC) 293 cells transfected with the ghrelin receptor GSH-R1a receptor (growth hormone secretagogue receptor) that the elevation of intracellular Ca2+ concentration induced by the stimulation of GSH-R1a receptor with ghrelin or its analog MK0677 is remarkably reduced in cells co-transfected with 5-HT2C receptor. They also showed in mice that ghrelin-stimulated food intake is attenuated by the 5-HT2C receptor agonist lorcaserin but augmented by the 5-HT2C receptor antagonist SB242084. More recently, they showed a mode of modulation of GSH-R1a receptor signaling by 5-HT2C receptor [30]. They showed that the stimulation of 5-HT2C receptor facilitates the heterodimerization of GHS-R1a receptor with 5-HT2C receptor and subsequent internalization, thereby resulting in an attenuation of GHS-R1a stimulation-induced elevation of intracellular Ca2+ concentration. Moreover, 5-HT2C receptor antagonist reverses the heterodimerization of GHS-R1a with 5-HT2C receptor, which restores the cellular response to the GHS-R1a agonist ghrelin. Huang et al. [31] also showed that atypical antipsychotics such as olanzapine, clozapine and risperidone induce weight gain and obesity by blocking 5-HT2C receptor and subsequent activation of GHSR1a signaling, in which reduction in dimerization of GHSR1a receptor with 5-HT2C receptor is involved.
These findings, taken together, suggest that not only the stimulation of ghrelin release by the antagonism of both 5-HT2B and 5-HT2C receptors but also the ghrelin receptor GHSR1a sensitization via blockade of 5-HT2C receptor contribute, at least in part, to the antiemetic action of olanzapine.
On the other hand, extensive care should be taken to avoid diabetic adverse events such as diabetic ketoacidosis, particularly in patients with pre-existing diabetes, since atypical antipsychotic drugs, including olanzapine, clozapine and risperidone, cause diabetic ketoacidosis [32,33]. The incidence of hyperglycemic emergencies, including hyperglycemia, diabetic ketoacidosis, hyperosmolar hyperglycemic state, is rare in non-diabetic patients (1-2 per 1000-person years), however, the rate is markedly elevated in patients with pre-existing diabetes (6-12 per 1000 person years) [34]. There are some differences in the rates of diabetic ketoacidosis associated with antipsychotic drugs: the rate of diabetic ketoacidosis is significantly higher in patients receiving olanzapine (0.107%: 55 cases/51,302 patients) than for risperidone (0.060%: 31 cases/51,330), in which adjusted relative risk is 1.62 (P=0.033) [35].
References
- de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, et al. (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76: 1055-1061. [Crossref]
- Rizk AN, Hesketh PJ (1999) Antiemetics for cancer chemotherapy-induced nausea and vomiting. A review of agents in development. Drugs RD 2: 229-235.
- Tavorath R, Hesketh PJ (1996) Drug treatment of chemotherapy-induced delayed emesis. Drugs 52: 639-648. [Crossref]
- Jordan K, Kasper C, Schmoll HJ (2005) Chemotherapy-induced nausea and vomiting: current and new standards in the antiemetic prophylaxis and treatment. Eur J Cancer 41: 199-205.
- Navari RM (2013) Management of chemotherapy-induced nausea and vomiting : focus on newer agents and new uses for older agents. Drugs 73: 249-262. [Crossref]
- Andrews PL, Sanger GJ (2014) Nausea and the quest for the perfect anti-emetic. Eur J Pharmacol 722: 108-121. [Crossref]
- Tan L, Liu J, Liu X, Chen J, Yan Z, et al. (2009) Clinical research of Olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 28: 131. [Crossref]
- Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A randomized phase III trial. J Support Oncol 9: 188-195.
- Iihara H, Fujii H, Yoshimi C, Yamada M, Suzuki A, et al. (2015) Control of chemotherapy-induced nausea in patients receiving outpatient cancer chemotherapy. Int J Clin Oncol 8: 16215-16222.
- Nawa-Nishigaki M, Kobayashi R, Suzuki A, Hirose C, Matsuoka R, et al. (2018) Control of nausea and vomiting in patients receiving anthracycline/cyclophosphamide chemotherapy for breast cancer. Anticancer Res 38: 877-884.
- Navari RM, Nagy CK, Gray SE (2013) The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 21: 1655-1663.
- Vig S, Seibert L, Green MR (2014) Olanzapine is effective for refractory chemotherapy-induced nausea and vomiting irrespective of chemotherapy emetogenicity. J Cancer Res Clin Oncol 140: 77-82.
- Chiu L, Chiu N, Chow R, Zhang L, Pasetka M, et al. (2016) Olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a retrospective study. Ann Palliat Med 5: 172-178.
- Mizukami N, Yamauchi M, Koike K, Watanabe A, Ichihara K, et al. (2014) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind, placebo-controlled study. J Pain Symptom Manage 47: 542-550.
- Mukhopadhyay S, Kwatra G, Alice K P, Badyal D (2017) Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized controlled study. Support Care Cancer 25: 145-154.
- Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, et al. (2016) Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 375: 134-142. [Crossref]
- Hocking CM, Kichenadasse G (2014) Olanzapine for chemotherapy-induced nausea and vomiting: a systematic review. Support Care Cancer 22: 1143-1151. [Crossref]
- Wang XF, Feng Y, Chen Y, Gao BL, Han BH (2014) A meta-analysis of olanzapine for the prevention of chemotherapy-induced nausea and vomiting. Sci Rep 4: 4813. [Crossref]
- Chiu L, Chow R, Popovic M, Navari RM, Shumway NM, et al. (2016) Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 24: 2381-2392.
- Yoodee J, Permsuwan U, Nimworapan M (2017) Efficacy and safety of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: A systematic review and meta-analysis. Crit Rev Oncol Hematol 112: 113-125.
- Sutherland A, Naessens K, Plugge E, Ware L, Head K, et al. (Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults. Cochrane Database Syst Rev 9: CD012555.
- Shahid M, Walker GB, Zorn SH, Wong EH (2009) Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol 23: 65-73.
- Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, et al. (1999) Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res 37: 107-122. [Crossref]
- Lord CC, Wyler SC, Wan R, Castorena CM, Ahmed N, et al. (2017) The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest 127: 3402-3406.
- Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, et al. (2008) Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem 51: 305-313.
- Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, et al. (2008) Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterol 134: 2004-2013.
- Takeda H, Nakagawa K, Okubo N, Nishimura M, Muto S, et al. (2013) Pathophysiologic basis of anorexia: focus on the interaction between ghrelin dynamics and the serotonergic system. Biol Pharm Bull 36: 1401-1405.
- Hattori T, Yakabi K, Takeda H (2013) Cisplatin-induced anorexia and ghrelin. Vitam Horm 92: 301-317. [Crossref]
- Schellekens H, De Francesco PN, Kandil D, Theeuwes WF, McCarthy T, et al. (2015) Ghrelin's Orexigenic Effect Is Modulated via a Serotonin 2C Receptor Interaction. ACS Chem Neurosci 6: 1186-1197. [Crossref]
- Schellekens H, van Oeffelen WE, Dinan TG, Cryan JF (2013) Promiscuous dimerization of the growth hormone secretagogue receptor (GHS-R1a) attenuates ghrelin-mediated signaling. J Biol Chem 288: 181-191.
- Huang XF1,2,3, Weston-Green K1,3, et al. (2018) Decreased 5-HT2cR and GHSR1a interaction in antipsychotic drug-induced obesity. Obes Rev 19: 396-405. [Crossref]
- Vuk A, Kuzman MR, Baretic M, Osvatic MM (2017) Diabetic ketoacidosis associated with antipsychotic drugs: case reports and a review of literature. Psychiatr Danub 29: 121-135.
- Ely SF, Neitzel AR, Gill JR (2013) Fatal diabetic ketoacidosis and antipsychotic medication. J Forensic Sci 58: 398-403. [Crossref]
- Lipscombe LL, Austin PC, Alessi-Severini S, Blackburn DF, Blais L, et al. (2014) Canadian Network for Observational Drug Effect Studies (CNODES) Investigators. Atypical antipsychotics and hyperglycemic emergencies: multicentre, retrospective cohort study of administrative data. Schizophr Res 154: 54-60.
- Ramaswamy K, Kozma CM, Nasrallah H (2007) Risk of diabetic ketoacidosis after exposure to risperidone or olanzapine. Drug Saf 30: 589-599. [Crossref]

