Background: Less than 15% of acute respiratory infections (ARI) require antibiotics yet over 50% receive antibiotics.
Methods: FebriDx was performed on patients presenting with suspected ARI to an outpatient pediatric practice. Patients clinically suspected of bacterial infection received FebriDx testing and quantitative CRP. Physicians documented proposed treatment plan comparing the influence of using clinical assessment (CA) plus CRP versus CA plus FebriDx.
Results: Twenty children (2-12y) participated. Four had fever without source and 16 were clinically diagnosed with bacterial infection. Point-of-care (POC) testing plus CA determined that 90% (18/20) of patients originally characterized as bacterial by CA alone were subsequently deemed to have viral infections or microbiological unconfirmed respiratory illness (MURI) based on additional POC results. FebriDx reduced antibiotic prescription by 90% versus a 55% potential reduction associated with CRP. Patients did not require re-consultations or experience complications.
Conclusion: FebriDx may reduce unnecessary antibiotic prescribing compared to CRP testing in the outpatient pediatric setting.
respiratory infection, antibiotic stewardship, MxA, CRP, immunoassay; FebriDx, point-of-care test
Approximately 10-15% of presumed acute respiratory infections (ARI) are proven to be bacterial and require antibiotic therapy yet more than 50% of patients consistently receive antibiotic treatment [1]. The unnecessary prescription of antibiotics contributes to antimicrobial resistance [2,3] and leads to adverse drug events such as allergic reactions [4], diarrhea, secondary infections such as C. difficile infection [12] and return visits to a clinic. Furthermore, unnecessary antibiotic use significantly increases healthcare costs [5].
Fever is a common complaint seen in patients presenting to an outpatient pediatric clinic. In most cases, it is associated with acute respiratory tract symptoms including sore throat, earache, runny nose, or cough [6]. Most general practitioners (GPs) and pediatricians routinely depend on a clinical assessment for patients with a presumed diagnosis of otitis media, sinusitis, pharyngitis, and acute bronchitis [6]. It is challenging, especially in children in the early course of an infection, to accurately determine the etiology with a clinical assessment alone [7-10]. Diagnostic uncertainty and patient expectations frequently drive unnecessary antibiotic treatments [11]. For this reason, many European countries frequently perform in office C-reactive protein (CRP) testing to confirm bacterial infections.
Improving diagnostic certainty may help identify patients that will benefit from antibiotic treatment [5,12,13]. Studies have demonstrated that use of CRP in addition to the clinical assessment may safely reduce antibiotic prescriptions [14]. While elevated CRP is often associated with bacterial infections, viral infections such as adenovirus, parainfluenza virus, influenza, respiratory syncytial virus (RSV), herpes simplex virus (HSV), Ebstein-Barr virus (EBV) and herpes zoster virus can frequently stimulate a CRP ≥ 20 mg/L [15-21] and thus CRP is an imperfect biomarker to differentiate viral and bacterial infections. As a standalone biomarker, CRP has sufficiently high negative predictive value (NPV) and is thus useful for identifying a host response to a clinically significant infection, but it lacks the requisite specificity to distinguish bacterial from viral infection and therefore can lead to approximately 38-56% antibiotic overtreatment of patients presenting with acute respiratory symptoms and signs [22,23].
FebriDx is a 10 minute, point-of-care (POC) test that identifies a clinically significant pathogen- associated host immune response and differentiates viral from bacterial etiology in patients presenting with symptoms of ARI, through measurement of two biomarkers in whole blood fingerstick samples: Myxovirus resistance protein A (MxA) and C-reactive protein (CRP). MxA is an intracellular protein that becomes elevated in the presence of acute viral infections.
This pilot study aimed to determine if the initial diagnosis of bacterial infection and subsequent antibiotic prescribing decisions could be improved by the addition of POC testing such as standalone, quantitative CRP or FebriDx test for pediatric patients presenting with an acute fever.
Twenty pediatric patients presenting to a private pediatric practice in Switzerland during the winter of 2019 were prospectively evaluated as part of a pilot evaluation of the FebriDx test. Febrile pediatric patients presenting with symptoms consistent with febrile ARI and/or fever of unknown origin were clinically assessed by an experienced pediatrician for a bacterial or non- bacterial etiology. Only those patients clinically assessed, without access to POC testing, and determined to have a presumed bacterial infection based on symptoms and signs, were included in the pilot study. These patients were only evaluated with the POC tests when a participating physician was available and if it was decided that they required an antibiotic prescription without additional testing.
Clinical assessment included history and physical exam and a diagnosis of bacterial or non- bacterial cause was determined (initial classification). Patients with fever associated with a skin rash, rhinorrhea, and diarrhea were considered more likely to have a viral infection. Whereas, patients exhibiting fever as well as a sore throat associated with pus and enlarged cervical lymph nodes, or cough associated with age-based tachypnea, hypoventilation, indrawing and/or crackling rales, were presumed to have a bacterial infection. As part of routine clinical care, patients that met the clinical criteria for a bacterial infection underwent subsequent testing with a standalone quantitative CRP (QuickRead® 101, Orion Diagnostica, Finland) and dual biomarker FebriDx® (Lumos Diagnostics; Sarasota, FL, USA) testing (post-POCT classification). Quantitative CRP was performed on an analyzer according to the manufactures package insert. CRP values greater than 20 mg/L were considered significant for bacterial infection. The FebriDx test is an affordable, qualitative, single-use, disposable, fingerstick whole blood immunoassay with a turn-around time of 10 minutes [20]. The FebriDx test has an integrated all-in-one test cassette with a built-in retractable safety lancet, fixed volume blood collection and transfer tube, and push button buffer activation mechanism. The test requires 5 µL of whole fingerstick blood and provides a qualitative red test line result for elevated levels of MxA (C95 ≥ 40 ng/ml), a black test line result indicative for elevated CRP (C95 ≥ 20 mg/L), and blue control line to confirm a valid test result. A FebriDx test that results as elevated MxA, with or without an elevated CRP test line, is interpreted as a viral infection, the presence of any CRP test results without an associated elevated MxA, is interpreted as a bacterial infection and the presence of a blue control line without elevated MxA or CRP line is interpreted as a negative test.
Only patients who were presumed to have a bacterial infection, based solely on the pediatrician’s initial clinical assessment, were included in the evaluation. Considering that the clinical diagnosis prior to additional POC testing was ‘bacterial infection’, it was assumed without further testing, 100% of the patients clinically determined to have a bacterial infection would be prescribed an antibiotic. Thus, the ‘baseline’ antibiotic prescription rate prior to the standalone CRP and FebriDx testing was assumed to be 100% antibiotic prescribing decisions in patients with a clinical diagnosis of bacterial infection were reexamined with the addition of POC test based on the following conditions. For standalone quantitative CRP testing, all clinically suspected bacterial infections associated with a CRP ≥20 mg/L were confirmed as bacterial infections that warranted antibiotic treatment. CRP results of < 20 mg/L were considered to be either viral or lacking a clinically significant host response and thus antibiotics were deemed unnecessary and withheld. For FebriDx testing, any result showing an elevated CRP result line and a negative MxA result line was indicative of a host response to a bacterial infection and also warranted antibiotic treatment; however, a FebriDx test positive for MxA, with or without a positive line for elevated CRP, is indicative of a host response to a viral infection would not benefit from antibiotics. In order to assess if the addition of POC tests could impact antibiotic prescribing practices based solely on clinical diagnosis of
bacterial infection, single biomarker quantitative CRP and dual biomarker FebriDx were performed. All discrepancies between the POC test diagnosis and the clinical diagnosis as well as between the two POC tests were captured and the impact on potential treatment pathways was reported. Due to the higher sensitivity and specificity of the FebriDx test compared to standalone CRP, the treating physician used the FebriDx test results to confirm the final diagnosis and guide the prescription of antibiotic treatment if the results were discrepant to standalone CRP. Caregivers were instructed to call or return to clinic if the patient remained febrile or decompensated over the subsequent 2 days.
Ethical approval and informed consent
The treating pediatrician had access to all diagnostic test results at the time of the office visit. This pilot assessment provided the pediatrician with unblinded laboratory results with approved diagnostic and therapeutic procedures according to generally accepted standards of care at the time of therapeutic decision, for which the pediatrician had the ability to control any and all treatment decisions, and therefore, institutional review board approval was determined to be not required. Antibiotic prescriptions and absolute reductions were calculated for each clinical assessment and POCT scenario: (i) clinical assessment alone, (ii) clinical assessment + CRP, (iii) clinical assessment + FebriDx. Chi square test was performed to examine the addition of POC tests to clinical diagnosis on antibiotic prescribing.
Following routine clinical assessment, 20 febrile children were determined to have a presumed bacterial infection by solely the clinical assessment without any additional diagnostic testing. Of the 20 febrile children advancing to POC testing in the study, 20% (4/20) were determined to have an underlying bacterial infection presenting as a fever without a source and 80% (16/20) were presumed to have an acute febrile bacterial respiratory infection based on clinical diagnosis alone. All patients with a clinical diagnosis of suspected ‘bacterial’ infection subsequently had both a quantitative CRP and FebriDx test per formed (Figure 1).
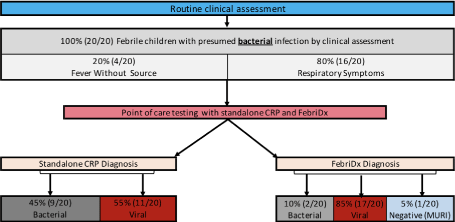
Figure 1. Presumed diagnosis of febrile patients – Standalone CRP vs FebriDx). MURI: microbiological unconfirmed respiratory illness
The ages of the children proceeding to POC testing ranged from 2 to 12 years, with a mean of 4.1 years. The tested population consisted of 12 girls and 8 boys.
POC testing with standalone CRP and FebriDx altered the presumed clinical diagnosis of bacterial infection. A bacterial infection was confirmed in patients with a CRP ≥ 20 mg/L or FebriDx demonstrating only a control line and CRP result line consistent with a bacterial infection. Patients tested with FebriDx and found to have a positive control line and a positive MxA result line, with or without a visible CRP result line, were classified as having a host response to a viral infection. If the FebriDx test showed a positive control line but was negative for both CRP and MxA result lines, this was deemed negative.
Standalone quantitative CRP testing demonstrated a CRP ≥ 20 mg/L in 50% (10/20) of the patients and were deemed a bacterial infection. FebriDx determined that 10% (2/20) patients to be a bacterial infection and further differentiated the other patients as viral in 85% (17/20) and microbiologically unconfirmed respiratory illness (MURI) in 5% (1/20) (Figure 1). Both patients found to have a positive CRP line on FebriDx without an associated elevated MxA, also showed a quantitative CRP ≥ 20 mg/L.
The intention to prescribe antibiotics according to clinical assessment alone was presumed to be 100%, whereby in the absence of standalone CRP or FebriDx, all patients (n=20) would have been treated with antibiotic based on history and clinical presentation. When considering a baseline of a 100% prescribing rate for clinical diagnosis alone, the addition of single biomarker CRP test results would have reduced physician prescribing by 50% (10/20) whereas the addition of dual biomarker FebriDx to clinical assessment actually modified 90% (18/20) of therapeutic decisions (Figure 2) p<0.00001. Follow up reveled resolution of clinical event with no further office consultations, antibiotics, or other complications.
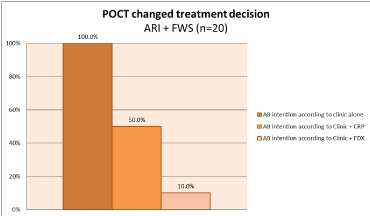
Figure 2. Impact of POCT on clinical decision to treat with antibiotics (AB) in all patients, ARI and FWS (n=20). FDX: FebriDx
Since 10% (2/20) of the initially suspected patients were confirmed as having a bacterial infection, 40% (8/20) antibiotic decisions were change by the addition of CRP to clinical assessment and 85% (17/20), p=0.77. of antibiotic decisions were change by the addition of FebriDx to clinical assessment (Figure 3).
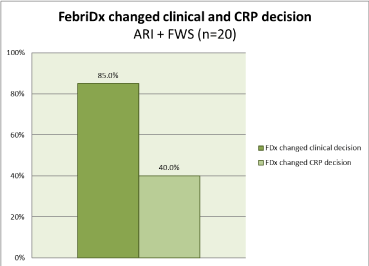
Figure 3. Impact of FebriDx on clinical and CRP decisions in all patients, ARI and FWS (n=20).
When only considering patients presenting with acute respiratory symptoms (n=16), the intention to prescribe antibiotics according to history and clinical assessment alone was presumed to be 100% (16/16). The intention to prescribe antibiotics using single biomarker CRP could have led to 43.8% (7/16) of patients receiving antibiotics whereas, dual biomarker FebriDx could have led to antibiotic prescription in 6.3% (1/16) of patients with a clinically suspected ARI p=0.01 (Figure 4).
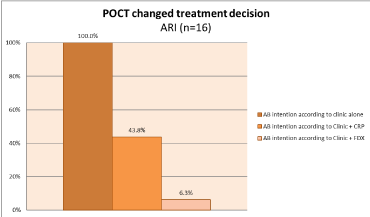
Figure 4. Impact of POCT on clinical decision to treat with antibiotics (AB), ARI only (n=16)
Therefore, when considering patients with symptoms of ARI, single biomarker CRP testing could have resulted in the avoidance of 56.3% of antibiotic prescriptions. Whereas, clinical assessment plus FebriDx avoided 87.5% of antibiotic prescriptions, p=0.049 (Figure 5).
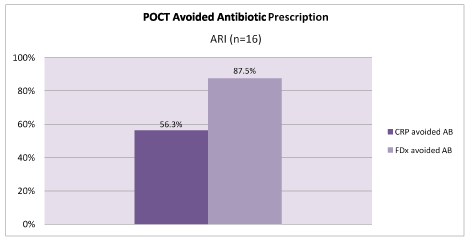
Figure 5. Antibiotics (AB) avoided by POCT in ARI patients only (n=16)
POC testing in the pediatric outpatient setting can significantly enhance diagnostic certainty, antibiotic prescribing, and thus support antibiotic stewardship efforts in patients with acute fever for suspected respiratory infection. In this pilot evaluation, both single biomarker, quantitative CRP testing and the dual biomarker FebriDx (MxA + CRP) improved the clinical diagnosis and supported a significant reduction in antibiotic prescription. MxA, is specifically induced by the production of Type I interferon (IFN) α/β which is a key component of the innate host response to viral infections and has immune modulating and antiviral functions [24]. MxA is not generally found to be elevated in healthy individuals nor expressed in response to cytokines associated with bacterial infections such as IL-1 or TNF-α [25-27] and therefore contributes to the much needed specificity to the interpretation of the single biomarker CRP test. Since lower CRP levels can be associated with both bacterial and viral infection [28], the addition of a viral host response biomarkers such as MxA allows lower CRP thresholds to be used, in order to prevent missing a clinically significant bacterial infection.
FebriDx testing combined with clinical assessment indicated that only 6-10% of patients warrant antibiotic treatment and 85-87.5% of patients would avoid antibiotics by incorporating the test results into the clinical decision. Using clinical judgement alone and no further POC testing, 100% of the patients would have received an antibiotic prescription (i.e. 20/20), however FebriDx could change the clinical inclination to prescribe antibiotics in over 85% of decisions compared to 40% for the standalone CRP testing. These data are consistent with findings in the adult, outpatient setting where a 48% of clinical decisions to prescribe antibiotics were impacted by FebriDx which resulted in an 80% reduction in antibiotics [11]. Also, similar to the adult study, all patients in the current study, inclusive of patients who received or did not receive antibiotics, made a full recovery and did not require additional office visits or antibiotics.
The FebriDx test was well tolerated by the pediatric patients as the participating children did not cry (or report pain) during the fingerstick portion of the test. Although the test incorporates all components into one, portable, easy to use cassette, collecting and transferring the blood requires complete filling of the capillary collection tube or blood will not transfer to the test strip because of lack of capillary action and lead to invalid tests. There was some initial learning required to master this step.
One symptomatic patient was negative for both elevated MxA and CRP on the FebriDx test and was confirmed to have a quantitative CRP level less than 8 mg/L and thus no antibiotics were prescribed. This patient represents a group of patients who present symptomatic but lack a clinically significant host immune response and are often found to exhibit a microbiologically unconfirmed respiratory illness (MURI). MURI patients make up between 28-62% of patients that present with symptoms and signs of acute respiratory infections depending on whether or not the patients are confirmed febrile at the time of presentation [23]. Patients with microbiological confirmation that lack a systemic immune response suggests the existence of microbial colonization or a clinically insignificant local infection. Those patients without microbial confirmation and a limited immune response may represent a self-limiting infection or convalescent phase, or represent a case of MURI and likely do not require immediate antibiotics and may be managed through a watchful waiting strategy [17,23]. Both the identification of (i) a pathogen in association with a systemic immune response or (ii) a systemic host immune response without pathogen identification, not only suggests the presence of a clinically significant infection but also may guide antibiotic prescriptions.
The other borderline patient case included a quantitative CRP test that was >160 mg/L and FebriDx test that was elevated for both CRP and MxA. The vast majority of patients (>98-99%) with an elevation in both MxA and CRP are eliciting a host response to a viral infection [17,22,23]. Despite the POC determination of a viral infection, due to the CRP level >100 mg/L, a bacterial co-infection could not be ruled out. The decision, based on the age of the patient, was to treat the patient as a possible co-infection. After one day of observation the patient was managed as a probable viral infection.
The incidence of co-infection for ARI, inclusive of otitis media, sinusitis, pharyngitis, and bronchitis, is generally low. True co-infections with a confirmed host immune response as opposed to co-identification of a pathogen occurs in less than 1%-2% of cases [17,23,29-32]. Furthermore, it is well documented that viral infections, such as influenza, Epstein-Barr virus and adenovirus, may lead to substantial increases in CRP, often 100-172 mg/L [17,33-35] and thus a CRP > 100 mg/L is possible in a patient with a viral associated host immune response.
Although FebriDx cannot differentiate a rare co-infection, the 97-99% NPV suggests that the FebriDx test may support an initial watchful waiting antibiotic strategy [23]. Notwithstanding, antibiotics are often prescribed with the mindset of ‘just in case’ to avoid missing a potentially serious bacterial infection. However, in the context of low risk patients with acute respiratory infections (ARI), the risk of developing a serious complication due to lack of antibiotic prescription is very low as evidenced by the evaluation of 3.36 million episodes of ARI presenting to UK primary care practices to determine the extent to which antibiotics reduce the risk of developing serious complications. Over 4000 cases would need to be treated with antibiotics to prevent one serious complication resulting from the primary ARI [36]. Despite the low risk of developing complications and the high NPV of FebriDx for identifying a bacterial infection [23], the need for antibiotics should be strongly considered in vulnerable populations such as neonates and immunocompromised patients.
This study has several limitations including the small sample size, single centre experience, and the lack of blinded reference test results. Although the finding of statistical significance should be interpreted in the context of the small sample size, the data is promising and supports pursuing larger outcome studies, inclusive of more diverse patient populations.
POC testing may significantly improve antibiotic stewardship in the outpatient setting. FebriDx test results improved clinical management decisions and resulted in a significant reduction in antibiotic prescriptions. POC diagnostic testing with the FebriDx test may provide a cost-effective approach to outpatient antibiotic stewardship.
No funding or sponsorship was received for this study. The FebriDx tests were donated to Terres Des Hommes to support the pilot study.
No funding was provided for this evaluation.
Author declares no financial interest or conflict of interest.
- Pouwels KB (2018) Actual versus ideal ™antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother 73: 19-26. [Crossref]
- ECDC E (2009) The bacterial challenge: time to react. Stockholm: European center for disease prevention and control.
- Guillemot D, Courvalin P, French R (2001) Working party to promote research to control bacterial, better control of antibiotic resistance. clinical infectious diseases: Infectious Diseases Society of America 33: 542-547.
- Grijalva CG, Nuorti JP, Griffin MP (2009) Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. Jama 302: 758-766. [Crossref]
- Mainous AG, Hueston WJ (1998) The cost of antibiotics in treating upper respiratory tract infections in a medicaid population. Archives of Family Medicine 7: 45-49.
- Macfarlane J (2001) Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the community. Thorax 56: 109-114.
- De Sutter A (2006) Predicting prognosis and effect of antibiotic treatment in rhinosinusitis. The Annals of Family Medicine 4: 486-493. [Crossref]
- Hopstaken RM (2005) Clinical items not helpful in differentiating viral from bacterial lower respiratory tract infections in general practice. J Clin Epidemiol 58: 175-183.
- Lindbaek M, Hjortdahl P, Johnsen UL (1996) Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults. BMJ 313: 325-329. [Crossref]
- Metlay JP, Kapoor WN, Fine MJ (1997) Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. Jama 278: 1440-1445.
- Davidson M (2017) FebriDx point-of-care testing to guide antibiotic therapy for acute respiratory tract infection in uk primary care: A retrospective outcome analysis. J Infect Dis Preve Med 5: 165.
- Flanders SA (2004) Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. The American journal of medicine 116: 529-535.
- van der Meer V (2005) Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ 331: 26.
- Cals JW, Ebell MH (2018) C-reactive protein: guiding antibiotic prescribing decisions at the point of care. Br J Gen Pract 68: 112-113. [Crossref]
- Engelmann I, Dubos F, Lobert PE, Houssin C, Degas V, et al. (2015) Diagnosis of viral infections using myxovirus resistance protein A (MxA). Pediatrics 135: e985-993. [Crossref]
- Halminen M (1997) Expression of MxA protein in blood lymphocytes discriminates between viral and bacterial infections in febrile children. Pediatric research 41: 647-650.
- Joseph P, Godofsky E (2018) Outpatient antibiotic stewardship: a growing frontier-combining myxovirus resistance protein a with other biomarkers to improve antibiotic use. Open Forum Infect Dis 5: 20-24. [Crossref]
- Kawamura (2012) New sandwich-type enzyme-linked immunosorbent assay for human MxA protein in a whole blood using monoclonal antibodies against GTP-binding domain for recognition of viral infection. J Clin Lab Anal 26: 174-183. [Crossref]
- Nakabayashi M (2006) MxA-based recognition of viral illness in febrile children by a whole blood assay. Pediatric research 60: 770-774.
- Sambursky R, Shapiro N (2015) Evaluation of a combined MxA and CRP point-of-care immunoassay to identify viral and/or bacterial immune response in patients with acute febrile respiratory infection. Eur Clin Respir J 2: 28245. [Crossref]
- Sarov I (1982) Serum amyloid A levels in patients with infections due to cytomegalovirus, varicella-zoster virus, and herpes simplex virus. J Infect Dis 146: 440-443. [Crossref]
- Self WH (2017) Diagnostic accuracy of FebriDx: a rapid test to detect immune responses to viral and bacterial upper respiratory infections. J Clin Med 6: 90-94. [Crossref]
- Shapiro NI (2018) A prospective, multi-centre US clinical trial to determine accuracy of FebriDx point-of-care testing for acute upper respiratory infections with and without a confirmed fever. Annals of Medicine 50: 420-429.
- Goodbourn S, Didcock L, Randall RE (2000) Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol 81: 2341-2364. [Crossref]
- Simon A (1991) Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines. J Virol 65: 968-971. [Crossref]
- Calandra T (1990) Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon- alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis 161: 982-987.
- Girardin E (1988) Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med 319: 397-400.
- Falk G, Fahey T (2009) C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Family practice 26: 10-21.
- Lingard H, Zehetmayer S, Maier M (2008) Bacterial superinfection in upper respiratory tract infections estimated by increases in CRP values: a diagnostic follow-up in primary care. Scand J Prim Health Care 26: 211-215.
- Tsalik EL, Henao (2016) Host gene expression classifiers diagnose acute respiratory illness etiology. Sci Transl Med 8: 322ra11. [Crossref]
- Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, et al. (2015) Community-acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med 373: 415-427. [Crossref]
- Mäkelä MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, et al. (1998) Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 36: 539-542. [Crossref]
- Virkki R, Juven T, Rikalainen H, Svedström E, Mertsola J, et al. (2002) Differentiation of bacterial and viral pneumonia in children. Thorax 57: 438-441. [Crossref]
- Vasileva D, Badawi A (2019) C-reactive protein as a biomarker of severe H1N1 influenza. Inflamm Res 68: 39-46. [Crossref]
- Matesanz JL (2003) Plasma Procalcitonin and C-Reactive Protein Concentrations in Pediatric Patients with Epstein-Barr Virus Infection. Clinical chemistry 49: 2103-2104.
- Petersen I (2007) Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ 335: 982.





