For in vitro cultural purposes, cytokines and a variety of other synthetic constituents have been used in time past to support the growth of Hematopoietic Stem Cells (HSCs) and many other critical human cells. However, in the case of HSCs, it has not been possible to ensure their long-term survival and expansion by merely using cocktails of these cytokines. Endothelial Cells (EC), the main type of cells lining the blood vessels, lymphatic vessels and the heart are favorable to HSC growth. When in co-culture with HSCs, ECs have been found to be key in supporting HSCs in vitro. Harvesting sufficient number of endogenous ECs is critical for these cells’ utility in experiments and for clinical purposes. The number of natural endogenous ECs obtainable by most current procedures fall short of that required for clinical application and HSC co-culture purposes; hence researchers have now resorted to the use of synthetic brand of ECs which have its own shortcomings. Although good for experimental purposes, it may not be easily adaptable for clinical therapy. This review is intended to blend the current technologies in use for therapy to formulate a procedure for obtaining large numbers of endothelial cells that could be used in co-culture with HSCs or even for use in clinical therapy.
hematopoietic stem cells, expansion, endothelial cells, culture media, stromal vascular fraction, ultrasonic cavitation
Endothelial cells have been cited as being of great utility in the clinic, having shown good potentials in vascular regeneration therapy. Specifically, these unique cells promote the growth of new vessels when used in the treatment of myocardial ischemia, critical limb ischemia and in bone regeneration; in addition to benefits obtained in tissue engineering applications [1]. The ECs stimulate repair in ischemic tissues by expressing angiogenic, vasculogenic and paracrine effects on the affected tissues. ECs release nitric oxide that enables smooth contraction and relaxation of blood vessel muscular tissues. Endothelial cells also offer another benefit of not being tumorigenic when transplanted.
In transplantation, the number of cells effecting repair in a damaged tissue is critical to effective healing. Halcox et al. [2], found that there is a strong correlation between cardiovascular risk factors and the number of endothelial progenitor cells. The number of endothelial cells available for therapy and even for experimental purposes is usually limited, although the demand for cell therapy involving endothelial cells is quite high. Expandable alternatives like embryonic stem cell ECs (hESC-ECs) and induced Pluripotent Stem Cell ECs (iPSC-ECs) produced from host blood vessels represent viable options for therapy [3]. Reed et al. for instance, advocated for a source of endothelial cells that could be stored centrally and that could be used the same way as drugs [3]. There is no doubt that scientists are still far away from that goal. The type of cell therapy being advocated would involve the use of transplantation. Being derived endogenously and from autologous sources, these cells would serve both purposes.
Several tissue sources exist from which endothelial cells may be obtained. These sources include placental tissue, skin, Wharton’s jelly (the waxy coat on newly born babies), brain, omentum, retina, endometrium and the synovium. Human Vascular Endothelial Cell (HUVEC) fraction, also known as vascular endothelial fraction from blood vessels, bone marrow stromal vascular fraction (bone marrow) etc. [3,4] are other possible sources. In harvesting endothelial cells from these sources, the Endothelial Progenitor Cells (EPC) are often harvested along with the mature endothelial cells. The EPCs have been characterized as expressing the CD34+ HSC marker as well as VEGF-receptor 2 and AC133. CD34 is not exclusively expressed on hematopoietic stem cells; consequently, CD133 may better be used as a marker for the immature stem cells. Higher vascular regeneration potential has been demonstrated in CD133+/CD34- EPCs in comparison to CD133+/CD34+ EPCs. Moreover, CD133+/VEGFR2+ cells are more like immature EPCs, whereas CD34+/VEGFR2+cells represent the more matured Endothelial Cells (ECs) [5].
These cells have also been demonstrated as having capability to repair damaged blood vessels in non-diseased as well as in diseased states using in vivo experiments [6]. Until recently, the commonest source of endogenous endothelial cells for experimental purposes allogeneic cells that is not only tumorigenic, but that can also not be as efficacious as endogenously-derived endothelial cells.
This review has put together a protocol for sourcing abundant numbers of endothelial cells that could be used in the clinic both for therapy and for experimental purposes without the risk of tumor. Indeed, the initial goal was to formulate a procedure for harvesting a large population of endothelial cells for use in co-culture with hematopoietic stem cells as to have sufficient number for autologous has been the human microvascular endothelium from excised tissues. Presently, the most feasible source for obtaining sufficient amount of endothelial cells for clinical use or expansion of HSCs with relative ease is the stromal vascular fraction from fat tissues; although currently, few investigators have explored this option. Stromal Vascular Fraction (SVF) is a heterogenous cellular product comprised of the mononuclear cells derived from adipose tissue and it is known to contain not only endothelial cells, but also T regulatory cells, monocytes, mesenchymal progenitor/stem cells, preadipocytes, pericytes and even hematopoietic stem cells plus a variety of other cells [7]. Essentially, SVF is adipose tissue deprived of the mature adipocytes. Adipose tissue contains a variety of growth-supporting cells and has become a veritable and attractive cell source in tissue engineering and regenerative medicine because it can easily be collected and enriched for its stem/progenitor cell populations. SVF-derived mesenchymal progenitor/stem cells can be easily expanded in vitro and have the potential to create diverse lineages of cells [8]. SVF as a source of stem cells for transplantation was first described by Zuk and colleagues who identified mesenchymal-like stem cells in SVF that could be induced to differentiate into adipogenic, chondrogenic, myogenic, and osteogenic lineages [9]. Since then, other studies have demonstrated a high content of Endothelial Progenitor Cells (EPC) in adipose tissue [10,11]. Indeed, the use of SVF already has wide application in clinics [12-14]. Processing the fatty tissue to obtain the vascular or stromal cell fraction which consists of stem and progenitor cells is a means of concentrating the desired cells and doing away with mature adipose cells that have little or no value in transplantation. It is not until the fatty tissue has been processed in the laboratory that SVF is obtained. According to an educational research website, endothelial cells (mature and progenitors) represent between 7% and 30% of SVF and ASCs are less than 10%, depending on which protocol is adopted in the fractionation process. Fibroblasts could represent up to 50% of SVF while CD34+ cells are present in large numbers and could comprise up to 63% of SVF (Figure 1).
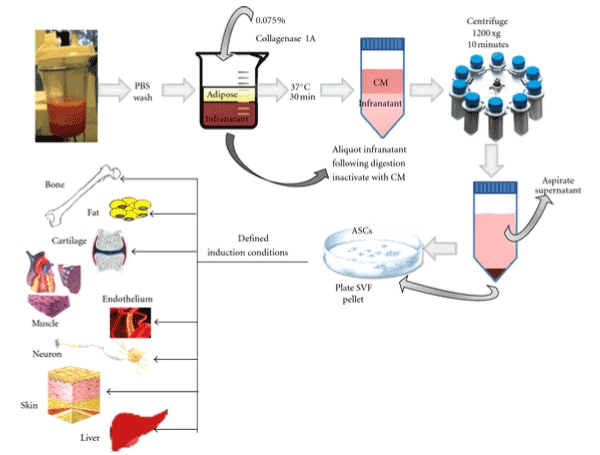
Figure 1. A graphical illustration of the processing of fatty tissue by enzymatic method to obtain Stromal Vascular Fraction (SVF)
Processing of autologous fatty tissue by various methods yields SVF primary cells containing sufficient endothelial and stromal cells that may be used clinically and experimentally. Endothelial cells derived from the SVF are termed adipose-derived, endothelial cells. Functional demonstration of adipose EPC has been performed in experiments in which SVF was purified for CD34 positive cells. SVF contains cellular activity capable of stimulating angiogenesis and conditioned media from SVF has stimulatory effect on host angiogenesis. There are reports that EPC in the SVF is capable of stimulating angiogenesis directly or through release of growth factors such as IGF-1, HGF-1 and VEGF [15,16]. SVF has been used in recent times to re-vascularize infarcted body tissues with considerable success [12-14]. One great advantage of SVF over other sources of endothelial cells is that in addition to having cellular constitution similar to cells that make up the microenvironment of the bone marrow, fat tissue from commonly used sites is easier to obtain than samples from the bone marrow and any of these other sites. Moreover, the presence of endothelial cells and fibroblasts makes it an ideal source for clinical use or the expansion of HSCs ex vivo.
There are two types of vascular fraction products obtainable from fatty tissue. The first type is known as Stromal Vascular Fraction (SVF). The other type is termed Vascular Fraction Cells (VFC) (the type produced by Intellicella for Regen Med) which is a good starting material for culturing HSCs with or without further selection. This is because the VFC and even the SVF are both rich in stromal cells and those other niche cells required for boosting HSC growth and proliferation. However, the VFC may contain more endothelial cells than is found in the SVF, being essentially sourced from the vascular portion that is first separated from the fatty tissue before processing, rather than the whole of fat tissue. On the contrary, SVF would have more of Adipose Stem Cells (ASCs) because the ASCs are embedded within the actual fat tissue. Vascular tissues are known to be rich in endothelial cells because the endothelium of blood vessels is constituted majorly of endothelial cells. Therefore, vascular tissues from various sites have been targeted whenever the need to isolate or source endothelial cells for certain reasons becomes necessary. Since the two fractions obtained from processing fatty tissue contain a heterogeneous population of cells similar to that obtained from the bone marrow stromal fraction, we may term them the Vascular-Stromal Cells (VSCs). Numerous protocols exist for autologous collection and administration of SVF which contains all of the cellular elements mentioned above and its clinical use has been proven to be safe [13,17]. The historical goal of harvesting SVF was to obtain sufficient Adipose Stem Cells (ASCs) for use in cosmetic surgery, tissue (cartilage, muscle and bone) repair and regeneration [18,19]. However, since the cell population is heterogeneous, it may be adapted to other clinical and laboratory uses.
For most clinical uses, purified ECs are not necessary and SVF or VFC would suffice except for purposes of determining the number of ECs contained in the vascular fraction. However, in the case of HSC culture, some level of purification may be necessary. Stromal cells generally and endothelial cells in particular, are essential for the long-term survival and proliferation of HSCs. Butler et al. [20] found out that in stroma-free cultures, there is a rapid attrition of HSCs, suggesting that the cross-talk between HSCs and BM stromal or vascular cells may be necessary to support long-term self-renewal and maintenance of HSCs in the niche. Importantly, the endothelial cells from the SVF and the VFC are derived largely from the microvasculature of the autologous fatty tissue and it has been recognized that Microvascular Endothelial Cells (MEC) are more appropriate than large vessel ECs that was initially used, since angiogenesis involves MEC rather than large vessel ECs [4]. It is pertinent to mention that stromal vascular fraction has also been isolated from the bone marrow, and some other anatomical sites [21]. This is not surprising since blood vessels and especially the microvasculature traverse the whole length of the human body system. The only snag in sourcing vascular cells from some of these other sites is the risk of exposure to highly invasive but avoidable procedures in the process of obtaining the microvascular tissue, with potential adverse complications. In addition, on a volume basis, the cell yield in fat tissue is between 100 and 1000-fold higher in frequency than that of bone marrow and the volume obtainable in a single procedure in the latter is quite restricted [17]. On the contrary, the fatty tissue obtained from the belly site is relatively painless with little or no complication. Moreover, a patient’s autologous stem cells processed from fatty tissue may be re-infused back or used in expanding his/her own HSCs. Briefly, about 60cc or more of adipose tissues are removed from the body, usually from the belly or back area, via surgery or liposuctioning under local anesthesia (the belly site has generally been preferred).
It may also be collected via ultrasound-assisted liposuctioning [5]. The volume required depends on the intended use. However, for the purpose of HSC expansion, a minimum of 100cc of adipose tissue may be required, if there is no intention to first expand the ECs before use in co-culture. Up to 500cc may be collected and sample screening and processing is carried out in a sterile flow hood, inside of a class 10,000 clean room [22]. In the protocol being suggested, primary cells only are used for the expansion of HSCs. Fatty tissue from donors who are less than 50 years may serve better since Masuda et al. [23] and Aird et al. [24] found that aging affects endothelial cells’ angiogenic function. It is not yet known however, if the aging effect applies in the in vitro setting. The next stage involves digestion of the fat tissue to obtain the vascular-stromal cells.
Presently, isolating the stromal vascular fraction from adipose tissue may be carried out through a variety of methods. These include the manual enzymatic and the manual mechanical [16]. There are also the automated versions of both the enzymatic and the mechanical. In addition to all these, there is one other current method known as ultrasonic cavitation. In the manual enzymatic method, enzymes are used to digest the fatty tissue to break the extracellular matrix and the bonds that hold the tissue together. The enzyme preparations used to achieve this purpose include dispase, trypsin and more commonly collagenase. Collagenases are endopeptidases that digest native collagen, the major fibrous component of the extracellular matrix. Bacterial collagenases are often used because they have broad substrate specificity. Tissue dissociation enzymes usually consist of mixtures of type I and type II collagenases isolated from Clostridium histolyticum, and various other proteolytic enzymes such as neutral protease which is known commercially as Dispase [25,26] isolated from P. polymyxa or thermolysin [27,28] isolated from G. stearothermophilus or B. thermoproteolyticus; depending on the product used. The freshly harvested lipoaspirate is washed with sterile 1% PBS until golden in colour. All visible blood and excessive fluids are eliminated, in order to get the fat to be as clean as possible. The washed fat is then placed in a magnetic stirrer for 1hr at 37°C. This is with a view to homogenizing it. The adipose tissue is then digested with 0.01% collagenase/PBS solution at a ratio of 1 ml of enzyme solution to 1 cc of adipose tissue. This mixture is incubated at 37°C with intermittent agitation until it becomes cloudy (usually about 30 minutes). The infranatant is then carefully aspirated, transferred to 50 ml conical tubes and centrifuged at 700g for 8 minutes. The supernatant is discarded and resulting pellet, the SVF, is resuspended in control media - DMEM supplemented with 10% FBS or 20% autologous serum, 500 IU penicillin and 500 μg streptomycin (autologous serum is to be preferred to fetal bovine serum as the latter may expose the SVF to xeno-contamination). The cells are then counted and adjusted to a concentration of 1 × 106 cells per cc [6,29]. In using stromal cells for HSC culture, the use of enzymes and chemicals may be detrimental if no further step of purification is undertaken, unless the washing of pellet is very thorough. Other methods are also available for harvesting SVF from fatty tissues, which are well documented with their cost-benefit analysis in Aronowitz et al. [26]. The method used by Regen/ Intellicela is not only automated, it appears to be best suited for the purpose of harvesting large numbers of vascular-stromal cells. The technology is known as ultrasonic cavitation. No other simpler method currently exists, neither is there any other better source from which large numbers of endothelial cells may be obtained with such ease. While the ease of Peripheral Blood (PB) collection may be a little better, enriched endothelial progenitor cells from the peripheral blood is less than 0.01% of the PBMNC population [23], as compared with a minimum of 7% in SVF. Even in the bone marrow, EPCs constitute only 0.1% of BMMNC [23] whereas harvesting cells from the bone marrow requires an invasive, painful, and time-consuming procedure, while the cell yield is not comparable to that from fat tissue. There is however the question of whether the number of primary cells obtained from SVF will be sufficient for large scale expansion of HSCs, an issue that is addressed later in the text.
A number of studies have shown that better digestion of the extracellular matrix is achieved with proteolytic enzymes than by mechanical methods [17,26]. Proteolytic enzymes are proteases that are capable of breaking the bonds that hold peptide molecules together. In this case, the enzyme breaks down the extracellular matrix responsible for holding the adipose cells firmly together, thus releasing the cells. Enzyme treatment by this biochemical mechanism therefore confers a greater measure of efficiency to the separation process. Mixtures of enzymes have been preferred to the use of single enzymes because the former yields more nucleated cells than using only one enzyme, owing to the synergistic effect of the proteolytic enzymes in the breakdown of the extracellular matrix [29,30].
On the other hand, the breakdown of the cells by mechanical methods is an alternative non-enzymatic means of removing the SVF cells from the adipose tissue [31] and tends to involve washing and shaking or vibrating the lipoaspirate, in a vibrating shaker for 6 min at 600 vibrations /minute, followed by centrifugation at 1600 rpm for 6 minutes with a view to concentrating and isolating the SVF cells. The step of shaking and vibrating tissue is a modification of the original procedure in which Human Umbilical Vein Endothelial Cells (HUVEC) or the Microvascular tissue Endothelial Cells (MEC) that replaced it later are obtained by the mincing of vascular tissue manually with the aim of releasing the vessel endothelial cells through physical shearing. This is then followed by the centrifugation step. Optimal cell recovery is better achieved with a centrifugation speed of 1200 g [32]. Most non-automated mechanical methods available contain a centrifugation step which main purpose is to concentrate the SVF cells after shearing. In addition, the stem and progenitor cells sediment by density while the less-dense upper layer consisting of mature adipose cells is discarded. The composition of the cell populations recovered through simple centrifugation and other non-enzymatic methods have been shown to contain a greater frequency of Peripheral Blood Mononuclear Cells (PBMNC) and a substantially lower number of progenitor cells [26,33-35]. This constitution seems more favorable to clinical transplantation of SVF than for cell expansion since the T regulatory cells have no role in cell culture but are required for proper engraftment of transplanted Adipose Stem Cells (ASCs). Nevertheless, a great plus for the mechanical procedure is the enhanced number of endothelial cells and the significant reduction in the number of ASCs which are not required in large numbers in HSC co-culture. Reports indicate that this physical separation mechanism rather than the enzymatic digestion may yield as much as 95% mixture of endothelial cells and traces of other blood cells (majorly the mononuclear cells). As a matter of fact, less than 10% of the SVF obtained by the mechanical method are ASCs, the rest being essentially endothelial cells, red blood cells and blood mononuclear cells [35]. In growing HSCs, endothelial cells are the paramount feeder cells required, although other stromal cells have synergistic booster effect on HSC growth. Even the presence of trace adipocytes is beneficial to the proliferation of HSCs [36]. The heterogeneous nature of SVF bodes well for the purpose of using these cells as feeder cells for HSC expansion, provided the larger fraction is composed of ECs, as no further purification may be necessary. In addition, since expensive enzymes are not employed in digestion, the cost factor is significantly reduced with the manual mechanical processing.
Without further selection, using high-yielding harvesting procedures, enough cells may be available to use for co-culture with HSCs from the harvested SVF. Published yields of viable, nucleated SVF cells achieved using manual, collagenase-based digestions range from 100,000 nucleated cells /cc to 1,300,000 nucleated cells/cc of the lipoaspirate processed [26]. However, lower total yields of cells are usually obtained with manual mechanical methods. It would be logical to take advantage of the relative high yield of endothelial cells with the mechanical method for the purpose of using harvested SVF for HSC co-culture. To obtain greater yields of endothelial cells, efficient automated mechanical methods may be required.
The use of automated systems is necessitated by the need to keep the lipoaspirate sterile, a purpose which most of these systems succeed in achieving. In addition, they save valuable time and reduce the tedious manual procedures. There are evidences as well that show greater cell yields per cc of lipoaspirate than the manual procedures [17,26]. Some of the automated systems available commercially also make use of the enzyme digestion methods. Many of them employ proprietary enzymes for their systems. They include the Cellution system from Cytori Inc. which makes use of the Cellase enzyme; the Icellator Cell Isolation system from Tissue Genesis that makes use of the Adipase enzyme blend; Biosafe America’s Sepax system which is another enzymatic and fully closed system produced in Switzerland and the GID SVF system, a fully closed system that is capable of being used as a single-use, disposable system with its proprietary enzyme, GIDzyme -2. The yields among these automated systems vary. For instance, the Cellution system is capable of yields ranging from 240,000 to 360,000 nucleated cells per cc of lipoaspirate and it can process up to 360cc of fatty tissue at a time. On its part, investigators have found that TissueGenesis’ Icellator is capable of a yield around 700,000 nucleated cells per cc of fatty tissue. Data regarding this machine has not been consistent. The Sepax technology is reported to yield about 260,000 nucleated cells per cc, while the GID SVF system can process 350cc of lipoaspirate at a time, with a yield of 719,000 nucleated cells per cc (Table 1).
Table 1. Comparison of methods used in obtaining Vascular Stromal Cells (VSCs)
Method
|
Mode of action
|
Advantages
|
Disadvantages
|
Manual
|
Manual mincing of fat tissue with release of ECs and other cells through shearing. Cells are then concentrated by centrifugation
|
The initial method known for processing vascular fraction cells from vascular tissue.
No other advantage.
|
Tedious, cumbersome, time-wasting. Cells are prone to contamination. Product consists mainly of mature mononuclear cells
|
Mechanical
|
Mechanical mincing with release of ECs through shearing followed by centrifugation
|
Improvement on the manual method. Saves considerable time. A greater percentage (up to 95%) of cell product is ECs. Devoid of washing process and the use of chemicals. Cost reduction
|
Cell composition is not truly heterogeneous as most are either mature mononuclear cells or ECs. Less total cell yield in fraction than is obtainable with enzyme techniques
|
Enzymatic method
|
Proteases break down the extracellular matrix that holds the cells together. Cells are thus free in suspension.
|
Comparable efficiency. Greater total cell yield than mechanical method
|
Several washing processes. Product is prone to contamination and loss of valuable cells. Makes use of chemicals which may not be thoroughly removed by washing. Relatively expensive
|
Automated systems
|
A combination of mechanical procedures meant to operate in automated fashion so as to enhance efficiency
Synchronized into a system
|
Sterility is fairly assured. Improvement on the mechanical method and better ease of operation
|
Expensive machines required. Cell yield may vary, depending on the manufacturer. Some make use of enzymes in application, thereby further increasing cost
|
Ultrasonic cavitation
|
Homogenization of adipose tissue by ultrasonic cavitation, followed by centrifugation and separation by density
|
Very high yields of cells. Composition of fraction is fairly constant and so is predictable. More ECs are expected than in mechanical method. Free from additives
|
Expensive, although may not cost more than some automated systems. Use of the technology still uncommon
|
On the contrary, automated mechanical systems are few. Except perhaps for the purpose of sterility, most of the processes required in automated mechanical harvesting of SVF are possible with standard laboratory equipment [26]. One of the automated systems, StromaCell™ is a mechanical device that may be operated in a closed sterile system to which the lipoaspirate may be introduced directly. It yields about 140,000 nucleated cells per cc of fatty tissue. Another device that has been used for long in harvesting and homogenizing adipose tissue for endothelial cells was described by Hu et al. [27], which is capable of obtaining 1.12- 2.13 × 106 cells that are larger than 7.8 μm from 1 g adipose tissue but only after enzymatic isolation of the non-enzymatic isolated cells. Manually, an increased population of microvascular endothelial cells can be collected with an elongated cannula with cutting edges to disrupt the connective tissue. It is nonetheless clear that the number of stem and progenitor cells obtained from most of these procedures is quite inadequate to sustain large scale growth of HSCs in culture. Going by the Butler et al. [20] experiment, a ratio of forty ECs to one HSC is required at the seeding stage, implying that if billions of HSCs are to be produced, large numbers of ECs are also required to serve as feeder layer in culture. Ultrasonic cavitation is a novel automated system that yields very large numbers of SVF cells. This technology yields SVF consisting of a functionally diverse population of cells that is believed to be synergistic in action when used clinically.
Ultrasonic cavitation is a patented technology initially used in removing fat from the body of individuals desiring to enhance their cosmetic looks. It has now been adapted for processing high numbers of stem and progenitor cells in the Stromal Vascular Fraction (SVF) technology. The technology is able to dissociate the fat cells and the blood vessels contained within the adipose tissue to bring out the stromal vascular fraction after a centrifugation process [38]. The step by step procedure involves:
Desired volume of the lipo-aspirate is obtained by liposuction. Fatty tissue is transferred to a sterile container. Fixed ultrasonic amplitude/power applied to fatty tissue causes oscillations within the cell, resulting in the contents of the adipose tissue being emulsified. Continued exposure also causes the mature adipose cell membrane to rupture. Further use of the ultrasonic probe causes the emulsification of the released fat from the mature adipose cells and water content to occur. The exposure to the ultrasonic probe may take between thirty and sixty minutes. It is necessary to monitor the temperature at this stage to ensure it is not too high as to adversely affect the desired cells. By this time, homogenization of the adipose tissue has occurred and after a centrifugation step, separation of desired cell pool is achieved (Figure 2).
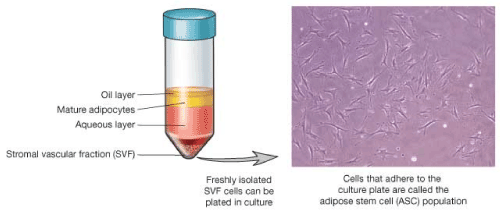
Figure 2. Diagrammatic representation of processed SVF layers in a centrifuge tube (source: Regenerate US)
After the ultrasonic cavitation process, the fat cells are removed. This is made possible by using the centrifugation process, by which the density of components causes layering. This then allows one to selectively collect or dispose off a portion of the material. The stem cells which are in the SVF layer are collected along with the extra- cellular matrix which is labeled as the aqueous layer in the diagram. This is now ready for cell analysis and preparation for use or storage.
This process generally will produce a higher number of cells compared to manual processes which use enzyme or chemical processes. This may be due to the fact that the process maintains a more constant and complete cell composition throughout, as the need to wash the added chemical or enzymes out of the sample is avoided, a step which results in a significant loss of valuable cells. In addition, all the valuable cells are spared from attrition by this process. The process is 100% free from additives and uses only the automated (ultrasonic) mechanical method of separation. It should be remembered also that more endothelial cells are shed into the SVF per volume by the mechanical process than by enzymatic process, thus ensuring abundant availability of endothelial cells desired for the co-culture of HSCs.
The Regen Medical Centre is one facility that makes use of cellular products processed from patients who have made autologous donations of their fatty tissue so as to yield cell products after it must have been processed in the laboratory. One of the cell products made from donated fatty tissue is vascular fraction cells, consisting of pluripotent adult stem cells, endothelial cells, progenitor cells, fibroblast cells, RBCs, WBCs and growth factors contained in the vascular tissue. The processing is carried out by a partner laboratory, Intellicella Biosciences, a laboratory dedicated to processing stem cells from tissues.
The procedure involves obtaining 60cc of adipose tissue from the belly of patient by liposuction under local anesthesia. The network of capillaries surrounding the adipocytes is removed by ultrasound probe and the vascular tissue is washed in a sterile area, spun in a centrifuge at low speed for a long time, thus permitting the autologous vascular cells to sediment to the base of the container; before testing for quality.
Quality testing is carried out using a flow cytometer which determines cell count and viability. All the processing is completed in one hour and the final cell product is placed in an intravenous (IV) bag for onward transport to the clinic, for treatment to commence.
The Vascular Fraction Cell (VFC) product so obtained is commonly used in the treatment of patients suffering from osteoarthritis, a disease with high incidence in the United States. VFC also known as Adipose Derived Stem Cells (ADSC) may also be helpful in the treatment of multiple sclerosis, myocardial infarction, stroke, type II diabetes, autism, for dermal regeneration and for aesthetic purposes. Being autologous cells, Intellicella’s VFC administration to the patient carries no risk of allergy or rejection. It also complies with the FDA requirement of minimal manipulation since cell expansion is not attempted and the use of serum, enzymes and bovine agents which could endanger the patient to risks of xeno-contamination is avoided. Sterility is also reasonably assured since the processing is automated. These processes align with Good Manufacturing Practice (GMP) for cell products meant for administration to humans. One major advantage of using VFC for treatment is the variety of cells involved. With the many types of cells in VFC, growth factors and cytokines required to stimulate repair to any type of disease would be made available by the appropriate stem cells. However, because the cells obtained to make this product are shed entirely from the vascular network of capillaries during centrifugation, a larger proportion of these cells would be endothelial cells and fibroblast cells. The actual percentage of each type of cell that constitutes the VFC is not yet known but it is expected to vary slightly from one individual to another.
The other type of cellular product available for processing by Intellicella is known as Stromal Vascular Fraction (SVF). The process of obtaining SVF is similar to that of VFC with slight modification. In the case of SVF, the whole fatty tissue from the donor-patient is subjected to centrifugation after being broken down with the ultrasound equipment, unlike in the VFC in which the vascular tissue portion is first separated for processing. After obtaining the SVF, quality checks are also carried out. SVF contains cytokines and growth factors; in addition to a variety of progenitor cells, stem cells and blood cells derived from the capillaries supplying the fat cells and the extracellular matrix in the exorcised fatty tissue. Some of the growth factors contained in SVF include TGF-beta, PDGF, FGF, among others. These growth factors along with the cellular elements stimulate tissue recovery in a paracrine manner, when delivered into an injured or diseased tissue, thus serving as chemoattractants, for the recruitment of more endogenous stem cells to the diseased tissue site. The stem cells so infused then differentiate appropriately into the required cell lineage to effect tissue regeneration. In addition, SVF cells might provide free radical scavengers, anti- oxidant agents as well as chaperone/ heat shock proteins that mitigate against apoptosis of the surviving cells.
Aside the Regen Medical Center earlier mentioned; another center that practices the use of SVF in treatment of patients is the California Stem Cell Treatment Center which is part of the Cell Surgical Network that engage strictly in the use of autologous stem cells to carry out investigational studies on their patients. According to this video (https:// youtu.be/JqGLsSvtVXk?t=122), they avoid the use of allogeneic bone marrow, umbilical cord blood or placenta in the treatment of their patients. By employing the use of autologous adult stem cells obtained from fatty tissue by liposuction, the personnel are able to treat a wide range of diseases, especially degenerative diseases, cardiopulmonary and neurological disorders and orthopedic cases. Liposuction is a minimally invasive procedure that takes less than ten minutes but yield more cells per treatment than many other tissue sources. While admitting that the procedure is not yet FDA-approved, their treatment studies have been subjected to review by an Institutional Review Board (the exact technology for processing SVF was not stated). In addition, a university’s faculty collects their data for analysis.
Fatty tissue collected for processing is subjected to ultrasonic cavitation for about thirty minutes. This period is adequate for the blood vessels and the fat cells to explode or lyse. The technology makes use of a probe that explodes the mature fat cells while sparing the stem and progenitor cells. The blood vessels thus release the stromal vascular fraction cells contained within the outer layer of their blood vessel walls without adversely affecting the viability of isolated cells. Indeed, very high numbers of these cells are released by this method because in causing mature adipose cells to explode and lyse, the ultrasonic cavitation process also causes the blood vessels to explode and release all the cells contained within the endothelium, a feat not achievable by other processes currently in use. No stem or progenitor cell is trapped within the tissue that would not be released. This technology is thus able to yield ten times the number of cells that is obtainable by other existing methods. After the ultrasonic probe exposure, the fatty tissue is centrifuged and the layer of exploded fat staying at the topmost layer is removed and discarded [37,38]. Using appropriate buffers, the cells may now be washed and counted with a flow cytometer or desired cell types may be selected with the flow cytometer, since each cell type possesses specific antigens by which it may be identified and isolated.
The yield of cells from this technology runs to billions, although there may be individual variations [39]. Normally the cell concentration is similar in healthy adults and a possible cause in slight variation in numbers could be due to the actual size of the fat cells from an individual. They could be larger in size, occupying more of the volume of extracted material. Average amount of fat tissue commonly used per patient is 150cc and the average number of cells per cc is between 9 and 12 million cells. This gives an estimated total of about 1.5 billion cells.
For clinical use, the processed SVF cells obtained are utilized as follows: Two IV infusions of 300 million cells are given to the patient followed by one local (site) injection of 200 million cells. It may be necessary to give multiple injections in order to give therapeutic effect, if much lower number of cells is used in therapy [14]. The patient is allowed to heal from the liposuction for one week. When treatment is due, the cells are thawed and rinsed with PBS and Human AB serum, diluted in saline solution and autologous serum, loaded into sterile syringes, and then transported in a controlled-temperature cooler for onward delivery (accompanied by the corresponding certificate) to the physician for therapy [14]. In other words, about a billion cells are required and sufficient for patient treatment. Left-over cells bottled in vials, each consisting of about 250 million cells per vial, are cryo-preserved in liquid nitrogen for probable use of the patient in the future.
In comparison to other automated systems currently in use, the ultrasonic cavitation technology appears superior in terms of cell yields per cc. For instance, the best systems - whether enzymatic or mechanical, yields about a million cells per cc., whereas the ultrasonic method yields an average of ten million cells per cc., a ten-fold increase in yield. By multiplying, the total number of cells that could be obtained when using a modest 100cc of fatty tissue, the cell yield runs to at least a billion and according to Rodriguez et al [14], collection of up to 600cc of fat tissue is possible from an individual. It is safe therefore to say that this technology is very efficient for processing adipose tissue into SVF, making enough ECs available and rendering the expansion of endothelial cells before use in co-culture of HSCs, unnecessary.
SVF from fatty tissue contains diverse cells of the stromal bone marrow. In vivo, SVF has been used to support the growth and repair of several tissues, including HSCs [12-14]. While some investigators have used SVF in MSC ex vivo expansion, there is yet to be documented, any attempt to use SVF for in vitro expansion of HSCs. This may be because historically, SVF was intended for use in the repair of tissues with adipogenic origin. Moreover, aside the HSCs, Adipose Stem Cells (ASCs) are one of the dominant cells in SVF, making up to 10% of the SVF fraction. Endothelial cells are also co-dominant in SVF. In this regard, the Vascular Fraction Cells (VFC) processed by the method outlined above, offers a greater potential of harvesting more endothelial cells that are naturally abundant in blood vessels. Particularly large numbers of endothelial cells may be obtained by the mechanical processing of the vascular fraction of the fatty tissue with the ultrasound technology. After harvesting VFC or SVF from fatty tissue using the cavitation process, cells are suspended in endothelial cell (transport) buffer - Endothelial Basal Medium (EBM) MV supplemented with 20% autologous serum, 100 U /100 μg/ml Penicillin/ Streptomycin, sodium heparin (10 μg/ml) and basic fibroblast growth factor (bFGF, 2.5 ng/ml) [40]. Gargett et al. [4] found that human serum was essential for EC’s growth, hence the addition of autologous serum. There is also a type of basal medium for endothelial cells by Sigma-Aldrich that is serum-free and chemically defined. If selection for pure Endothelial Cells (ECs) becomes necessary, there are some commercial kits made for this purpose. Endothelial cells may be selected by the use of antibodies to CD31, using these kits. A few of the kits for endothelial cell selection include the Dynabead 31 (Dynatech Inc.) and Microbead 31 (Miltenyi Biotec). Another method of isolating these cells so that ECs are purified is to place them in tissue culture in plastic. The pericytes and the adipose stem cells are separated based on adherence to plastic [41,42]. The non- adherent cells would essentially be endothelial cells along with a variety of other cells that are capable of supporting the expansion of ECs or HSCs. Since this method of selection is not based on use of cell markers, the endothelial cells would still be contaminated with plastic-adherent cells. Among the contaminating cells would be fibroblasts and Mesenchymal Stem Cells (MSCs), both of which are also good feeder cells for the growth of HSCs [43], although the yield obtained with endothelial cells as feeder cells is much higher when compared to the fibroblasts under the same culture conditions [21]. The presence of fibroblasts and traces of any stromal cells along with endothelial cells is not detrimental to the growth of HSCs in culture. For this reason, no other purification step may be necessary. If it is eventually decided that fibroblasts should be eliminated completely, there is a commercial product, the Human Vascular Endothelial PrimaCell™ system that includes an effective fibroblast elimination system, Vascular Endothelial FibrOut™ (www.chiscientific.com). It contains a mixture of cis-OH- proline, collagenase, D-valine, and formulated serum substitutes. This system can effectively remove Vascular Endothelial fibroblast contamination without affecting the behavior of the isolated endothelial cells. Counting of the isolated ECs is now made with a flow cytometer. However, where the number of endothelial cells obtained is found to be low after selection, expansion of ECs obtained therefrom becomes imperative. It is obvious though, that from the calculation of SVF cells obtainable from the ultrasonic cavitation process using about 100cc of adipose tissue, which yield runs into billions; even if only 10% of the SVF are endothelial cells, it would produce large numbers of ECs adequate for clinical use.
Characterization of endothelial cells may be carried out by a few procedures in the clinical setting. Based on endothelial cells’ ability to express ABH antigens [44-46], it is possible to characterize them with the lectin, Ulex Europeus (UEA 1). Ulex europeus A1 binding antigen may be detected on ECs grown on coverslips using 20 μg/ml biotinylated UEA-1 lectin for 30 minutes at room temperature [4]. An additional method of characterizing endothelial cells is application of the Matrigel tubular assay, in which ECs form a network of tubules on Matrigel. While other cell types can form networks in Matrigel, only ECs form tubes with lumens. The Matrigel assay is carried out by seeding cells (2.5 × 105) on 24-well plates precoated with growth factor-reduced Matrigel and incubated for 24 hours at 37°C to induce tubular network formation [6,46]. Yet another means of characterizing isolated ECs is by the migration assay. According to www.chiscientific. com manual on growth of primary ECs, vascular endothelial cells may be characterized by factor VIII production, angiotensin conversion, uptake of acetylated low-density lipoprotein as well as the presence of Weibel Palade bodies, apart from antigenic markers expressed on the cell surface. It is pertinent to add that there are commercial kits made ready for endothelial cell characterization that serve good purpose. Where facilities for flow cytometry are available, endothelial cells may also be characterized and isolated by the use of flow cytometer.
In spite of the large number of endothelial cells required for clinical and in vitro use, the required numbers may still be sourced as primary cells and if culture must be carried out for expansion, minimal manipulation would be required. The application of ultrasonic cavitation technology in fractionating fatty tissue product represents a viable option to solving the inadequacy of endogenous ECs for various applications. Most of the technologies cited are to enable availability of options. This protocol is however only adaptable in the autologous setting.
- Chong MS, Ng WK, Chan JK (2016) Concise Review: Endothelial Progenitor Cells in Regenerative Medicine: Applications and Challenges. Stem Cells Transl Med 5: 530-538. [Crossref]
- Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, et al. (2002) Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653-658. [Crossref]
- Reed, D. M., Foldes, G., Harding, S. E., & Mitchell, J. A (2013) Stem cell-derived endothelial cells for cardiovascular disease: a therapeutic perspective. Br J Clin Pharmacol 75: 897-906. [Crossref]
- Gargett CE, Bucak K, Rogers PA (2000) Isolation, characterization and long-term culture of human myometrial microvascular endothelial cells. Hum Reprod 15: 293-301. [Crossref]
- Kolaparthy LK, Sanivarapu S, Moogla S, Kutcham, RS (2015) Adipose tissue - adequate, accessible regenerative material. Int J Stem Cells 8: 21-127.
- Wong WT, Huang NF, Botham CM, Sayed N, Cooke JP (2012) Endothelial Cells Derived From Nuclear Reprogramming. Circ Res 111: 1363-1375. [Crossref]
- Mizer JC, Ichim TE, Alexandrescu DT, Dasanu CA, Ramos F, et al. (2012) Exogenous endothelial cells as accelerators of hematopoietic reconstitution. J Transl Med 10: 231. [Crossref]
- Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, et al. (2015) Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood 115: 957-964. [Crossref]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-229. [Crossref]
- Martínez-Estrada OM, Munoz-Santos Y, Julve J, Reina M, Vilaro S (2005) Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovasc Res 65: 328-333. [Crossref]
- Auxenfans C, Lequeux C, Perrusel E, Mojallal A, Kinikoglu B, (2011) Adipose-derived stem cells (ASCs) as a source of endothelial cells in the reconstruction of endothelialized skin equivalents. J Tissue Eng Regen Med 6: 512-518. [Crossref]
- Gimble JM, Guilak F, Bunnell BA (2010) Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther 1: 19. [Crossref]
- Lara-Hernandez R, Lozano-Vilardell P, Blanes P, Torreguitart-Mirada N, Galmes A, et al. (2010) Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann Vasc Surg 24: 287-294. [Crossref]
- Rodriguez JP, Murphy MP, Hong S, Madrigal M, March KL, et al. (2012) Autologous stromal vascular fraction therapy for rheumatoid arthritis: rationale and clinical safety. Int Arch Med 5: 5. [Crossref]
- Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, et al. (2004) Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110: 349-355. [Crossref]
- Sumi M, Sata M, Toya N, Yanaga K, Ohki T, et al. (2007) Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci 80: 559-565. [Crossref]
- Oberbauer E, Steffenhagen C, Wurzer C, Gabriel C, Redl H, et al. (2015) Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen (Lond) 4: 7. [Crossref]
- Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, et al. (2008) Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 34: 1178-1185. [Crossref]
- Kakudo N, Morimoto N, Ogawa,T, Kusumoto K (2014) Potential of Adipose-Derived Stem Cells for Regeneration Medicine: Clinical Application and Usefulness of Fat Grafting. J Stem Cell Res Ther 4: 204.
- Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, et al. (2012) Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood 120: 1344-1347. [Crossref]
- Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, et al. (2001) Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol 115: 186-194. [Crossref]
- Conde-Green A, Rodriguez RL, Slezak S, Devinder P, Goldberg NH, et al. (2014) Enzymatic digestion and mechanical processing of aspirated adipose tissue. Plast Recons Surg 134: 54
- Masuda H, Tanaka R, Fujimura S, Ishikawa M, Akimaru H, et al. (2014) Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and t lymphocyte to cells with regenerative potential. J Am Heart Assoc 3: e000743. [Crossref]
- Aird AL, Nevitt CD, Christian K, Williams SK, Hoying JB, et al. (2015) Adipose-derived stromal vascular fraction cells isolated from old animals exhibit reduced capacity to support the formation of microvascular networks. Experimental Gerontology 63: 18-26. [Crosseref]
- Fogarty WM, Griffin PJ (1973) Production and purification of the metalloprotease of Bacillus polymyxa. Appl Microbiol 26: 185-190. [Crossref]
- Aronowitz JA, Lockhart RA, Hakakian CS (2015) Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. SpringerPlus 4: 713. [Crossref]
- Ke Q, Chen A, Minoda M, Yoshida H (2013) Safety evaluation of a thermolysin enzyme produced from Geobacillus stearothermophilus. Food Chem Toxicol 59: 541-548. [Crossref]
- Banyard DA, Salibian AA, Widgerow AD, Evans GR (2015) Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med 19: 21-30. [Crossref]
- McCarthy RC, Breite AG, Dwulet FE (2010) Biochemical analysis of crude collagenase products used in adipose derived stromal cell isolation procedures and development of a purified tissue dissociation enzyme mixture.
- Breite AG, Dwulet FE, McCarthy RC (2010) Tissue dissociation enzyme neutral protease assessment. Transplant Proc 42: 2052-2054. [Crossref]
- Gimble JM, Shah FS, Wu X (2013) A non-enzymatic method for isolating human adipose-derived stromal stem cells. Cytotherapy 15: 979-985.
- Kurita M1, Matsumoto D, Shigeura T, Sato K, Gonda K, et al. (2008) Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg 121: 1033-1041. [Crossref]
- Shah FS, Wu X, Dietrich M, Rood J, Gimble J (2013) A non-enzymatic method for isolating human adipose-derived stromal stem cells. Cytotherapy 15: 979-985.
- Conde-Green A, Rodriguez RL, Slezak S, Devinder P, Goldberg NH, et al. (2014) Enzymatic digestion and mechanical processing of aspirated adipose tissue. Plast Recons Surg 134: 54.
- Raposio E, Caruana G, Bonomini S, Libondi G (2014) A novel strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg 133: 1406-1409. [Crossref]
- Glettig DL, Kaplan DL (2013) Extending human hematopoietic stem cell survival in vitro with adipocytes. Biores Open Access 2: 179-185. [Crossref]
- Hu CB, Myers KE, Peterson RC (2000) Devices for harvesting and homogenizing adipose tissue containing autologous endothelial cells. Patent 6020196A.
- Bright R, Bright P, Hansen B, Thomas W (2014) Isolation of stem cells from adipose tissue by ultrasonic cavitation, and methods of use.
- Victor S (2014) Isolation of stromal vascular fraction from adipose tissue obtained from postmortem source using ultrasonic cavitation.
- Unger RE, Peters K, Sartoris A, Freese C, Kirkpatrick CJ (2014) Human endothelial cell-based assay for endotoxin as sensitive as the conventional Limulus Amebocyte Lysate assay. Biomaterials 35: 3180-3187. [Crossref]
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, et al. (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy 15: 641-648. [Crossref]
- Silva KR, Liechocki S, Carneiro JR, Claudio-da-Silva C, Maya-Monteiro CM, et al. (2015) Stromal vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric ex-obese women. Stem Cell Res Ther 6: 72. [Crossref]
- Li N, Feugier P, Serrurrier B, Latger-Cannard V, Lesesve JF, et al. (2007) Human mesenchymal stem cells improve ex vivo expansion of adult human CD34+ peripheral blood progenitor cells and decrease their allostimulatory capacity. Exp Hematol 35: 507-515. [Crossref]
- Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52: 2745-2756. [Crossref]
- Appleby SL, Cockshell MP, Pippal JB, Thomson EJ, Barrett JM, et al. (2012) Characterization of a distinct population of circulating human non-adherent endothelial forming cells and their recruitment via intracellular adhesion molecules-3. PLoS One 7: e46996. [Crossref]
- Shetty P, Shah, K, Viswanathan C (2015) Understanding vascular Biology with the help of endothelial progenitor cells. Enliven: J Stem Cells Regen Med 2: 003


