Background: Manual circular staplers are a useful tool in creating anastomoses, but the inherent difficulty in applying a manual device to delicate tissue can compromise security and durability of the seal. This study was undertaken to determine the potential benefits of a novel ECHELON CIRCULAR™ Powered Stapler (ECP) compared to manual circular staplers.
Methods: ECP was compared to a commercially-available manual circular stapler for the operational functions of force-to-fire, tissue compression, and distal tip movement, and in porcine models of anastomosis for hemostasis, leak pressure, and tissue perfusion in the vicinity of the staple line. ECP was also evaluated in a porcine chronic survival study over 7 and 30 days to assess anastomotic wound healing macroscopically and microscopically relative to a manual circular stapler.
Results: ECP demonstrated 97% lower force-to-fire, 33% lower compressive forces and 37% reduced distal tip movement during application. There was no statistically significant difference in tissue perfusion between ECP and the manual device, although ECP displayed 61% less leaking at the staple line and 52% less bleeding at the cut line. There was no significant difference in anastomotic wound healing between ECP and a manual device at either 7 or 30-day survival time points.
Discussion: Because of the technical challenges of creating durable anastomoses, addition of power to a circular stapler may be even more important than adding power to a linear stapler. In both ex vivo and in vivo preclinical testing, ECP provided superior handling and ease-of-use, while creating strong, durable anastomoses with similar healing profiles to a manual device. Further research is necessary to determine whether these preclinical benefits have impact on patient clinical outcomes.
anastomosis, circular stapler, perfusion, leak pressure, ergonomics
Although the first recorded use of staplers in surgery occurred in 1827, wide-spread usage did not start until the development of linear staplers in the Soviet Union in the 1950’s [1]. Substantial improvements in stapling technology have been achieved, such as multi-row staple lines, switching from steel to titanium for superior biocompatibility, and most recently the introduction of powered devices.
Circular staplers to facilitate left-sided, particularly low, colorectal anastomoses first emerged in surgical practice in the 1970’s. Since that time there has been little improvement in the circular stapler function, although changes have been made to the staples and their configuration. Surgeons continue to experience difficulty in firing the devices [2,3], and this can lead to an unacceptably high rate of technical errors with manual circular staplers [4].
Powered staplers provide several benefits compared to traditional manual devices. They are easier to operate, especially for surgeons with smaller hands, but they also have technical advantages. Powered linear staplers have been shown to consistently produce fewer malformed staples [5,6]. The advantage of power has been shown to carry over to superior performance clinically [7-11] and even provide an economic benefit [12].
Based on geometric considerations, the technical challenges in creating a high-performing powered circular stapler are more daunting than for a linear device. Yet the same outcomes are desired, namely, an optimized stapling solution capable of reducing leaks without compromising perfusion. Tissue tension, poor blood supply and variable tissue thickness can compromise the anastomosis and lead to significant complications. Among complications, anastomotic leaks are a dominant surgical concern due to their high morbidity and mortality risks. Post-operative anastomotic leaks occur in up to 1%-4% of gastrectomies [13,14], 8%-11% of esophagectomies [15,16], and 6%-14% of colorectal resections, with mortality in the latter reported between 2% and 12% [17-20]. Post-operative anastomotic leak may result in 65%-81% higher total hospital costs and 56%-100% longer length of hospital stay [19,20]. Patients who had post-operative anastomotic leaks incurred an additional hospital cost of $28,600 [19].
Recently, the first powered circular stapler was introduced commercially, the ECHELON CIRCULAR™ Powered Stapler (ECP, Figure 1). This study was undertaken to evaluate the performance characteristics of ECP in both ex vivo and in vivo models, and to compare to other marketed manual circular staplers. Test measures included force to fire and compressive force, device tip movement during application, perfusion after stapling, leak pressure, hemostasis, and anastomotic seal integrity.

Figure 1. The ECHELON CIRCULAR™ Powered Stapler.
Devices tested were the ECHELON CIRCULAR™ Powered Stapler (CDH29P, Ethicon Inc., Cincinnati, OH), PROXIMATE® Curved Intraluminal Stapler (CDH29A, Ethicon Inc., Cincinnati OH) and DST Series™ EEA™ Stapler (EEA2835, Medtronic, Minneapolis MN). For all testing except for the survival study, comparisons were made between ECP and the DST Series™ EEA™ Stapler (DST). For the survival study, the comparisons were between ECP and the PROXIMATE® Curved Intraluminal Stapler (CDH).
Ex vivo testing
Force to Fire: The manual force required to fire the circular stapler devices was measured using a custom test rig incorporating a 500 lb load cell transducer monitored by TestWorks® software (MTS Systems Corporation, Eden Prairie MN). This measurement specifically assessed the operator’s force applied to the device at the time of firing.
Compressive Force: Force experienced by the compressed tissue was measured using a Tekscan Pressure Mapping System (South Boston, MA) using a 6230 sensor with a top pressure range of 300 psig and I-Scan software. Compressive forces were measured at 15 seconds after the device was closed to the lowest staple height setting on porcine intestinal tissue of double-wall thickness 1.7±0.2 mm.
Distal Tip Movement during Anastomosis: The test circular device was inserted rectally and advanced to the location of the transected colon. Test skins were mounted on the devices and the trocar was retracted to the lowest staple height setting. A sensor was mounted on the end effector. Tip measurement was recorded (Figure 2) as the surgeon fired the device using a trakSTAR™ 3D tracking system (Ascension Technology Corp, Shelburne VT 05482) and the total path distance was calculated.
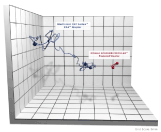
Figure 2. Tip movement during firing. Each cube represents a space measuring 5mm.
Leak Pressure: Porcine colon tissue with a flat width of 25-45 mm and double wall thickness of 1.3-1.9 mm was used to create anastomoses. Tissue thickness was determined by applying an 8 g/mm2 force for 15 seconds prior to measurement. Briefly, an anastomosis was created by loading colon tissue onto the casing of the device, cinching the tissue around the anvil stem with suture, compressing tissue for 15 seconds, and firing the device. Leak testing was performed using a metering pump (PULSAtron TL3011, Pulsafeeder, Punta Gorda, Florida) controlled by software (LabView, National Instruments, Austin, TX) to fill the anastomosis with water at a rate of pressure increase of 30 mmHg per minute for two minutes and a hold period at 60 mmHg for 15 seconds. The leak onset pressure was recorded together with total mass of leaked water. The proportion of leak pressures less than 30 mmHg were compared by Fisher’s Exact test and the median leak pressures were compared by a Kaplan-Meier log-rank analysis.
In vivo testing
The pig is generally accepted as the preferred large animal model for evaluation of anastomoses in the lower gastrointestinal tract [21], as it is monogastric and similar in anatomical features to humans. The in vivo procedures were reviewed and animals approved for use in the study by the Institutional Animal Care and Use Committee.
Anastomotic pressure testing: To determine relevant pressures for leak testing, a colorectal anastomosis was created in a porcine model. Briefly, the anastomosis was made by transecting the colon just above the pelvic brim with a linear stapling device and creating a purse-string at the proximal colon with a clamp and suture. The detachable anvil of a circular stapler was introduced into the bowel lumen and secured with the purse-string. The circular stapler was then inserted transanally and the anvil shaft was connected. The instrument was closed until the tissue was adequately compressed to a 1.5 mm staple height, and the device was fired.
After the anastomosis was created, the colon was occluded several centimeters proximal to the anastomosis and the pelvis was filled with saline. A video recording of the site was made as the air was insufflated into the bowel. Air insufflation was continued until the anastomosis and colon proximal to it were highly distended. During the injection, the air pressure was monitored and the values were synchronized with the video recording. Air pressure was monitored with a calibrated 5-psig pressure sensor (26PC Series Pressure Sensor 6BF6D, Honeywell, Morris Plains, NJ) and data acquisition system (DAQ, National Instruments, Austin, TX) interfaced with custom LabView software (National Instruments, Austin, TX). A total of 31 surgeons then gave independent evaluations of the selected video to determine maximal distension for clinically adequate leak testing.
Hemostasis testing: After a ventral midline abdominal incision in a porcine model, a gastrotomy was created in the posterior stomach wall near the greater curvature to facilitate anvil passage. The device was then applied to a single wall of gastric tissue. Tissue was compressed to the lowest staple height setting for 15 seconds, and the device was fired. Hemostasis was graded on a 5-point Likert scale [22], and the rate of bleeding was assessed quantitatively via hemoglobin assay. Blood for the hemoglobin assay was collected by blotting the staple line with gauze for 30 s and transferring to a pH 7.4 phosphate/EDTA buffer solution. The assay was performed spectrophotometrically using a hemoglobin colorimetric assay kit (Cayman Chemical, Ann Arbor, MI) and an Epoch spectrophotometer (BioTek, Winooski, VT) scanned under monochromatic light at a wavelength of 575 nm. Both the qualitative and quantitative measures of hemostasis were performed at 60 s and 300 s after firing. Comparisons between devices of Likert score and log-transformed hemoglobin levels were performed via ANOVA adjusted for time of sample evaluation.
Perfusion testing: Perfusion of blood in the peri-staple line area was measured using a Laser Speckle Contrast Imager [23-25] (moorFLPI-2, Moor Instruments, Wilmington, DE) to monitor the movement of blood cells. Internally developed software (MATLAB, MathWorks, Natick, MA) was used to extract and analyze the data for quantification of the perfusion at the site. Testing under conditions of maximal tissue compression was achieved by placing each device at the lowest setting within the recommended range on the staple height indicator of each device.
Each circular staple line was imaged at 300 seconds post-firing. The animal’s breath was suspended during imaging to minimize motion artifact. Tissue perfusion was quantified at four distinct regions within the circular staple line and perfusion was compared between the test and control devices at these locations. The areas selected for perfusion analysis included the following:
A: Non-compressed/non-stapled tissue, external to the outer row of staples.
B: Compressed tissue between the outer and inner rows of staples.
C: The inner-most ring of tissue near the cut edge, inside of the inner row of staples.
D: All compressed tissue within the staple line, from the outer row of staples to the cut edge.
The system had previously been validated by showing significant differences in perfusion between staples compressed at low (1.8 mm) and high (2.5 mm) staple height. For all locations except for the external ring (Location A), perfusion was significantly lower for the low staple height than the high staple height. The difference in perfusion between the low and high staple heights varied between a decrease of 20% within the staple region (Location B) to a decrease of 56% within the tissue flap area (Location C).
Survival Study: Both ECP and CDH were used to create colocolonic anastomoses in 21 female Yorkshire domestic pigs (including one replacement) weighing 35-54 kg. After surgical preparation and induction of anesthesia, a midline laparotomy was performed to expose the descending colon. The descending colon then underwent an end-to-end colocolonic anastomosis using either ECP or CDH. The middle of the descending colon was transected with endocutters and a purse-string was created in the proximal segment after the staple line was resected. The detachable anvil of the circular stapler was inserted into the bowel lumen and the anvil shaft secured with the purse-string. The circular stapler was inserted rectally and the anvil shaft and trocar were connected. Tissue was compressed to the lowest staple height setting and the instrument was fired. After removal of the stapler, a bubble leak test was performed by injecting at least 60 ml of air into the anastomosis and monitoring for leaks. Once the anastomosis was completed, the abdomen was closed in a standard fashion and the animal was recovered from anesthesia. Animals were survived and humanely euthanized at 7 days and 30 days. A necropsy was performed to evaluate the device insertion route (rectum up to anastomotic staple line) and to assess anastomotic healing. Anastomoses were harvested and tissue sections perpendicular to the anastomosis staple line were evaluated microscopically. The type and extent of tissue changes (e.g., inflammation, granulation tissue, fibrosis, etc.) was compared between devices.
Ex vivotesting: ECP had 97% lower force to fire during application than DST (p=0.001) and 33% lower compressive forces on tissue (p<0.001). Distal tip movement during application was decreased by 37% with ECP compared to DST (p=0.004, Figure 2). At bowel insufflation pressures of 30 mm Hg or less, ECP had 61% fewer staple line leaks than DST (p<0.001). The median leak pressure for ECP was 27% higher than for DST (p<0.001). All leakage pressures for ECP were greater than 20 mmHg, and all leakage rates for ECP were significantly lower than DST between 27 and 35 mm Hg (Figure 3) (Table 1).
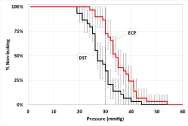
Figure 3. Kaplan-Meier survival plot with 95% confidence intervals for leak pressure comparing DST and ECP. The vertical line at 30 mmHg represents the primary comparison point.
Table 1. Comparisons between ECP and DST circular staplers.
Test |
ECP |
DST |
Statistical Test |
p-value |
Force to Fire
n
Mean ± St Dev |
30
1.92 ± 1.07 lbf |
3
80.7 ± 4.6 lbf |
Student’s t-test |
0.001 |
Compressive Force
n
Mean ± St Dev |
15
17.9 ± 3.0 lbf |
15
26.6 ± 4.8 lbf |
Student’s t-test |
<0.001 |
Distal Tip Mvmt
n
Mean ± St Dev |
15
57.6 ± 13.9 mm |
15
91.6 ± 40.2 mm |
Paired t-test |
0.004 |
Leakage Pressure
n
Leaks ≤ 30 mmHg
Median |
29
9/29 (31%)
33 mmHg |
29
23/29 (79%)
26 mmHg |
Fishers’ Exact
Log-Rank |
<0.001
<0.001 |
Hemostasis - Likert
n
Mean
Hemoglobin
n
Mean |
23
1.61
23
32.6 mg/dL |
23
2.59
23
68.4 mg/dL |
ANOVA
ANOVA |
<0.001
<0.001 |
Perfusion
n
Tissue Thickness
Blood Pressure
Location A
Location B
Location C
Location D |
24
2.27 ± 0.19 mm
70.0 ± 5.7 mmHg
392.7 ± 82.6
176.5 ± 62.6
3.9 ± 1.7
75.0 ± 32.3 |
24
2.19 ± 0.25 mm
67.8 ± 5.7 mmHg
418.3 ± 82.6
188.0 ± 37.3
4.1 ± 1.4
78.7 ± 19.7 |
Student’s t-test |
0.201
0.186
0.291
0.444
0.730
0.628 |
In vivo testing
Anastomotic leak pressure testing: All surgeons designated stop points within the 33-second length of the video. The pressure remained low during the initial filling and then rose quickly to a steady value. The diameter of the colorectum increased during the inflation at a decreasing rate. Distension first appeared at 5 seconds and all surgeon-selected stopping points were after 5 seconds. The mean pressure for the stopping points chosen by the surgeons was 26.0 ± 1.8 mmHg with a range of 23.9 - 32.5 mmHg.
Hemostasis: Hemostasis was significantly different between ECP and DST for Likert evaluation and hemoglobin assay (p<0.001 for both, Figure 4). On average, the ECP device demonstrated 52% less bleeding at the cut line than the DST device.
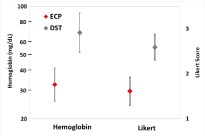
Figure 4. Hemostasis evaluated by hemoglobin assay and Likert scoring. Error bars represent two standard errors of the mean. Hemoglobin values are back log-transformed.
Perfusion: There were no significant differences in tissue thickness or blood pressure between the samples measured in the perfusion testing. There were no significant differences in rate of perfusion between ECP and DST at any of the locations evaluated (Figure 5). All differences in perfusion between ECP and DST were 7% or less. Application of a circular stapling device, regardless of device type, altered perfusion noticeably and predictably. Tissue perfusion was found to be markedly decreased as measurement location moved inward from the non-compressed tissue, toward the cutline. The non-compressed, non-stapled tissue, external to the outer staple row, was found to have the highest perfusion value (mean = 405.5 perfusion units, Location A) and served as a baseline perfusion measurement. The compressed tissue between the outer and inner rows of staples experienced a 55% reduction in perfusion (mean = 182.30 perfusion units, Location B). The compressed, inner-most ring of tissue near the cut edge and inside of the inner row of staples was found to have perfusion markedly reduced by 99% from the baseline value (mean = 4.01 perfusion units, Location C). Assessment of all compressed tissue within the staple line, from the outer row of staples to the cut edge provided an overall assessment of staple line perfusion based on the combined effect of tissue compression and a double row of staples. Tissue in this location experienced an 81% reduction in perfusion after circular stapler application (mean = 76.85 perfusion units, Location D), compared to the baseline non-compressed, non-stapled tissue.
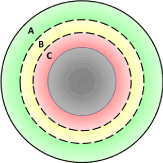
Figure 5. Perfusion measurement areas. A is non-compressed/non-stapled tissue external to the staples. B is compressed tissue between the staple lines. C is compressed tissue between the inner staple line and the cut edge. D is the combination of areas B and C.
Survival study: Intra-operatively, ECP was found to be equivalent to CDH with device insertion successful for all animals and the removal scoring acceptable for all animals at each interval. Postoperatively, one animal in the CDH group was euthanized three days after surgery due to declining clinical health and was replaced. Ultimately the cause of this animal’s decline was determined to be not directly related to the use of the stapler. All other animals, including one replacement, survived to the scheduled termination interval. Clinical observations, body weight, and clinical pathology data showed no differences between animals treated with the two devices at either interval.
Gross findings at anastomotic sites and device insertion routes were similar between groups at 7 and 30 days post-operatively indicating macroscopic tissue effects and healing for sites treated with the ECP were equivalent to those seen in animals treated with CDH. For the 30-day interval, the general condition of animals in both groups was good with the exception of one CDH animal that had a prolapsed rectum. This finding was not attributed to the stapler, but likely due to the surgical procedures.
Microscopically, ECP and CDH did not have any notable differences in terms of the tissue responses to healing (Figure 6). At the 7-day interval, at anastomosis sites, resected bowel ends were inverted into the colon lumen and layers of colon were bridged by minimal to mild granulation tissue infiltrated by macrophages, neutrophils and occasional rare eosinophils. The cut edges of all colon layers involved in the anastomosis were inverted into the lumen of the bowel and occasionally capped by fibrinonecrotic serocellular material. The degree of healing was similar in terms of mucosal epithelialization and was within expected limits for this early time point. At the 30-day Interval, resected bowel ends had minimal inversion into the colon lumen and layers of colon were bridged by minimal mature fibrous connective tissue. For both CDH and ECP, anastomosis edges had healed in near normal microanatomic relationship with mature minimal to mild fibrosis attended by minimal to no chronic inflammation and complete or almost complete epithelialization of the mucosal surface.
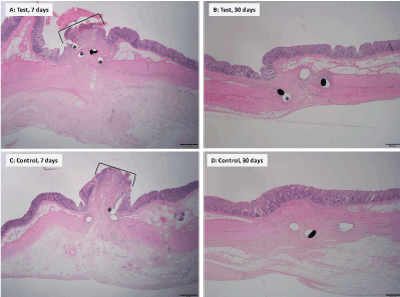
Figure 6. Illustrative histopathology images (hematoxylin and eosin stain) of anastomoses with the test and control devices at 7 days and 30 days post-operatively. 7 days (images A and C): Both test and control devices have a small mucosal epithelium gap (indicated by brackets). 30 days (images B and D): There is complete mucosal epithelialization and return to near microanatomic relationship in both test and control devices. Asterisks indicate staple voids and staples.
A key difficulty with the operation of a manual stapler is the force required to effect penetration of staples through tissue and form staples. Use of a manual circular stapler presents challenges beyond those encountered with a linear stapler, as evidenced by a study showing a high incidence of technical errors for a particular non-powered circular stapler during colon and rectal resections [4]. When applications were examined for misfiring, incomplete anastomosis and device failure, the rate of technical error was found to be 19%, with a rate of anastomotic error, such as inadequate donuts or staple line defects, of 9%. These errors were associated with a higher risk of gastrointestinal bleeding, transfusions and unplanned proximal diversions. Design changes in circular staplers could decrease the rate of technical errors and improve clinical outcomes.
One of these design issues is the force needed to fire the stapler. Studies have shown that some surgeons are simply unable to generate the grip strength force to fire circular staplers [2,3], and this may be an important factor in the aforementioned high rate of technical errors in their use to form anastomoses. The addition of power to the circular stapler has overcome the force to fire barrier in stapling. Reducing force to fire has a secondary benefit of stabilizing the device during firing, which can reduce distal tip movement. The powered operation also ensures a more consistent compression of both the staple and tissue, so that the average compressive force experienced by the tissue is lower than with a manual stapler. These benefits are in addition to the advantages of atraumatic Gripping Surface Technology that has separately been shown to provide gentler handling with a reduction in compressive forces on tissue and 3D Stapling Technology that evenly distributes compression throughout the anastomosis [5,11].
Perhaps the most desired feature in a circular stapler is reliability in creating anastomoses that are resistant to leak. Clinically, surgeons usually check the integrity of an anastomosis by performing a leak test, wherein the bowel lumen at the anastomosis is inflated with air and the serosal surface, under a pool of saline, is monitored for bubbles. Since surgeons typically inflate by hand, the actual maximum pressure used during testing is, in general, unknown. We developed a preclinical anastomotic leak test that indicates that the pressure inside an adequately inflated colon is approximately 26 mmHg. We believe that in clinical anastomotic testing pressures rarely exceed 35 mmHg in the bowel. One early study recommended a pressure of 25 cm saline (18.4 mmHg) as being sufficient to detect leaks while being within physiological limits [26]. Another study found that intrarectal pressure could not be raised above 35 mm Hg because of venting of air [27]. Insufflation is typically performed until the rectum is ‘optimally’ distended, which may be limited by air leak along the sigmoidoscope [28]. Based on these observations we have chosen 30 mmHg as a reasonable leak pressure criteria to assess the quality of a circular stapler in creating a secure anastomosis. In addition to providing a supra-physiological median leak pressure, ECP had 61% less leaking at pressures of 30 mmHg or lower than the non-powered circular stapler.
Although still a controversial topic [29], there is some indication that impaired tissue perfusion in the region of the staple line may be associated with dehiscence and anastomotic leakage, even when no air leak is detected during the procedure [30,31]. In our validated model, where we could detect perfusion differences between high and low-height staples, there was no significant difference in perfusion between ECP and the non-powered stapler at any site near or at the staple line. Hence, perfusion to the tissue for the ECP is similar to DST, even though hemostasis at the cut line was significantly better.
The initial strength of the anastomosis is important, but what is most critical is that the tissue heals producing a strong, durable connection. Intestinal wound healing has an initial lag period of several days where there is very low tissue strength, and then rapidly increases to approximately half-strength after 7 days with final strength occurring in 10 to 14 days [32]. Hence our final evaluation was an examination of anastomosis after survival periods of 7 and 30 days. At both time points we found that macroscopically and microscopically there was no difference in tissue healing between ECP and a manual circular stapler, confirming that the functional benefits observed with ECP do not negatively affect the healing response.
In these ex vivo and in vivo studies, we have demonstrated that design enhancements, including powered firing, can improve the function of the circular stapler. The ECHELON CIRCULAR™ Powered Stapler created secure anastomoses with higher leak resistance and less bleeding than manual circular staplers with no significant difference in tissue perfusion. Further studies are required to show that these benefits carry over from the preclinical to the clinical domain.
- McGuire J, Wright IC, Leverment JN (1997) Surgical staplers: a review. J R Coll Surg Edinb 42: 1-9. [Crossref]
- Kono E, Tomizawa Y, Matsuo T, Nomura S (2012) Rating and issues of mechanical anastomotic staplers in surgical practice: a survey of 241 Japanese gastroenterological surgeons. Surgery today 42: 962-972.
- Kono E, Tada M, Kouchi M (2014) Ergonomic evaluation of a mechanical anastomotic stapler used by Japanese surgeons. Surgery today 44: 1040-1047.
- Offodile AC, Feingold DL, Nasar A, Whelan RL, Arnell TD (2010) High incidence of technical errors involving the EEA circular stapler: a single institution experience. J Americ Coll of Surgeons 210: 331-335.
- Kimura M, Tanaka H, Hato M (2016) Evaluation of a New Stapler with Unique Surface Gripping Technology. Br J Med Medical Res 18: 6.
- Kimura M, Terashita Y (2016) Superior staple formation with powered stapling devices. Surgery for Obesity and Related Diseases 12: 668-672.
- Yoshimaru K, Matsuura T, Kinoshita Y, Hayashida M, Takahashi Y, et al. (2017) Graft reduction using a powered stapler in pediatric living donor liver transplantation. Pediatr Transplant 21. [Crossref]
- Ng CSH, Pickens A, Siegel JM, Clymer JW, Cummings JF (2015) A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devices. Euro J Cardio-Thor Surg.
- Licht PB, Ribaric G, Crabtree T (2015) Prospective Clinical Study to Evaluate Clinical Performance of a Powered Surgical Stapler in Video-assisted Thoracoscopic Lung Resections. Surgical technology International 27: 67-75.
- Qiu B, Yan W, Chen K (2016) A multi-center evaluation of a powered surgical stapler in video-assisted thoracoscopic lung resection procedures in China. J Thoracic Dis 8: 1007.
- Fegelman E, Knippenberg S, Schwiers M (2017) Evaluation of a Powered Stapler System with Gripping Surface Technology on Surgical Interventions Required During Laparoscopic Sleeve Gastrectomy. J Laparoendoscopic & Adv Surg Techniq 27: 489-494.
- Roy S, Yoo A, Yadalam S, Fegelman EJ, Kalsekar I, et al. (2017) Comparison of economic and clinical outcomes between patients undergoing laparoscopic bariatric surgery with powered versus manual endoscopic surgical staplers. J Medical Econom 20: 423-433.
- Oh SJ, Choi WB, Song J, Hyung WJ, Choi SH, et al. (2009) Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J Gastrointestinal Surg 13: 239.
- Giordano S, Salminen P, Biancari F, Victorzon M (2011) Linear stapler technique may be safer than circular in gastrojejunal anastomosis for laparoscopic Roux-en-Y gastric bypass: a meta-analysis of comparative studies. Obesity Surgery 21: 1958-1964.
- Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, et al. (2013) Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. The Annals of Thor Surg 96: 1919-1926.
- Markar SR, Arya S, Karthikesalingam A, Hanna GB (2013) Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann of Surg Oncol 20: 4274-4281.
- Trencheva K, Morrissey KP, Wells M (2013) Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Ann of Surg 257: 108-113.
- Schiff A, Brady BL, Ghosh SK, Roy S, Ruetsch C, et al. (2016) Estimated Rate of Post-Operative Anastomotic Leak Following Colorectal Resection Surgery: A Systematic Review. J Surg Surgical Res 2: 60-67.
- Hammond J, Lim S, Wan Y, Gao X, Patkar A (2014) The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg 18: 1176-1185. [Crossref]
- Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, et al. (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 148: 65-71. [Crossref]
- Bosmans JW, Moossdorff M, Al-Taher M, Beek L, Derikx JP, et al. (2016) Bouvy ND. International consensus statement regarding the use of animal models for research on anastomoses in the lower gastrointestinal tract. Int J Colorect Dis 31: 1021-1030.
- Siegel JM, Cummings JF, Clymer JW (2014) Reproducible, repeatable and clinically-relevant hemostasis scoring. J Adv Med Pharm Sci 1: 30-39.
- Tyrell J (2007) Video capability revives interest in laser method. Optics Laser Europe 147: 15-16.
- Stanhewicz AE, Ferguson SB, Bruning RS, Alexander LM (2014) Laser-speckle contrast imaging: a novel method for assessment of cutaneous blood flow in perniosis. JAMA Dermatology 150: 658-660.
- Milstein DM, Ince C, Gisbertz SS, Boateng KB, Geerts BF, et al. (2016) Laser speckle contrast imaging identifies ischemic areas on gastric tube reconstructions following esophagectomy. Medicine (Baltimore) 95: e3875. [Crossref]
- Gilbert JM, Trapnell JE (1988) Intraoperative testing of the integrity of left-sided colorectal anastomoses: a technique of value to the surgeon in training. Ann R Coll Surg Engl 70: 158-160. [Crossref]
- Beard J, Nicholson M, Sayers R, Lloyd D, Everson N (1990) Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. British J Surg 77: 1095-1097.
- Ivanov D, Cvijanovic R, Gvozdenovic L (2011) Intraoperative air testing of colorectal anastomoses. Srpski arhiv za celokupno lekarstvo 139: 333-338.
- Natoudi M, Theodorou D, Papalois A (2014) Does tissue ischemia actually contribute to leak after sleeve gastrectomy? An experimental study. Obesity Surgery 24: 675-683.
- Sherwinter D, Gallagher J, Donkar T (2013) Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorectal Disease 15: 91-96.
- Jafari MD, Wexner SD, Martz JE (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Amer Coll Surg 220: 82-92.
- Nelsen TS, Anders CJ (1966) Dynamic aspects of small intestinal rupture with special consideration of anastomotic strength. Archives of Surgery 93: 309-314.






