Patients on dialysis suffer from poor quality of life due to stringent dietary restrictions that limit food choices, decrease family and social interactions, and increase stress and anxiety. Sticking to these dietary restrictions is difficult, and patients’ struggles are compounded by large amounts of "hidden" phosphate additives in modern processed foods (e.g., frozen food, dry food mixes, packaged meat, cheese, and soft drinks) that are not required to be listed on labels. Current phosphate management strategies, including the use of phosphate binders, are insufficient to achieve and maintain target phosphate levels and increase the burden on patients to manage dietary restrictions. For the first time in decades, a new class of phosphate management options has been developed. New, non-binder therapies with novel mechanisms of action may more effectively maintain and achieve normal phosphate levels and allow patients to consume a more liberal diet containing healthy foods. One such therapy is a Sodium-Proton-Exchanger subtype 3 inhibitor that reduces intestinal phosphate absorption by targeting the dominant paracellular phosphate absorption pathway. This review will evaluate the benefits and negative impact of dietary restrictions for patients on dialysis, and discuss how recent novel therapeutic developments may present an opportunity for patients on dialysis to consume more healthy foods.
chronic kidney diseases, dialysis, dietary restriction, hyperphosphatemia, inorganic phosphate
Recommendation for dietary restrictions is standard of care
Dietary counselling is an established part of treatment for patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD), as well as for patients on dialysis [1]. As dialysis does not completely replace kidney function and can only remove a limited quantity of systemic waste [2], dietitians and nephrologists advise patients on dialysis to maintain a restricted diet (low phosphorus, potassium, sodium, liquids) to avoid the serious negative consequences associated with electrolyte overload (Table 1) [3-6]. Dietary electrolyte restrictions are also included in clinical guidelines: KDOQI/NKF and European Best Practice Clinical Guidelines recommend that patients on dialysis limit their phosphate intake to 800 - 1000 mg/day [7]. A dietary intervention to reduce phosphate intake lowered serum phosphorus by 1.67 mg/dL and 51% of patients in the intervention group achieved phosphorus concentrations <5.5 mg/dL vs. 18% of patients in the control group [8]. Lower serum phosphate is, in turn, associated with lower mortality risk [3]. However, a separate study found that prescribed dietary phosphate was not associated with improved survival in hemodialysis patients, and increased restriction may in fact be associated with greater mortality [9]. Dialysis centres are required by federal law to provide patients with counselling from qualified dietitians [10,11]. Dietitians and nephrologists focused on nutrition can help patients navigate dietary restrictions by providing practical, achievable recommendations. However, the multiple dietary restrictions recommended for patients on dialysis are difficult to achieve and may result in poor dietary quality [12]. This review will discuss the ways in which a restricted diet may lower patient quality of life, how processed foods make it difficult for patients to maintain a restricted diet, and the inadequacy of current phosphate management strategies to match dietary phosphate intake. The development of novel therapeutics that more consistently achieve target electrolyte levels may present an opportunity for patients to add a wider range of healthy foods to their diets.
Table 1
Electrolyte |
Negative Consequences |
Recommendations/ Healthy Levels |
Sodium |
Vomiting [85] |
Achieve and maintain: 135-145 mmol/L [87] |
Excessive sweating [85] |
Central nervous system dysfunction [85] |
Irritability and agitation [85] |
Lethargy, somnolence, and coma [85] |
Orthostatic hypotension and tachycardia [85] |
Brisk reflexes and myoclonus [85] |
Subarachnoid or subdural hemorrhage [85] |
Phosphate |
Increased overall mortality in adults with and without CKD [88] |
Achieve and maintain: 2.5-4.5 mg/dL [99] |
Increased cardiovascular morbidity and mortality [89,90] |
Vascular calcification [91] |
Hypertension [92] |
Increased FGF23* [93], and PTH# [94], levels |
FGF23*: Left ventricular hypertrophy [95] and congestive heart failure [96] |
PTH#: Increased inflammation [97] and coronary microvascular impairment [98] |
Fluid |
Swelling, cramping, bloating [100] |
Avoid fluid accumulation >10% of body weight [99] |
High blood pressure [100] |
Shortness of breath [100] |
Increased cardiovascular morbidity [100] |
Congestive heart failure [101] |
Pulmonary edema [101] |
Delayed wound healing [101] |
Tissue breakdown [101] |
Impaired bowel function [101] |
*FGF23, fibroblast growth factor 23; #PTH, parathyroid hormone
Dietary restrictions negatively impact patient quality of life
Many patients on dialysis do not consume a heart-healthy diet [13]. The negative impacts of dietary restrictions on the quality of patients' diets are 2-fold: patients can't consume the long list of healthy foods that may increase the risk of electrolyte overload, and patients must adhere to dietary restrictions continuously, likely depriving them of foods they enjoy. Thus, dietary restrictions for patients on dialysis lead to monotonous meals that are likely low in nutritional content.
Dietary restrictions also negatively impact patients' quality of life by limiting family and social interactions. Purchasing different ingredients to prepare separate meals than those eaten by other members of the household is difficult, expensive, and time-consuming. Consuming a different meal than everyone else increases feelings of isolation, potentially causing withdrawal from family interactions. Additionally, patients on restricted diets feel as if they are unable to participate in social activities with colleagues or friends because these activities are often centred around food. Patients may feel that they can’t adhere to dietary restrictions at restaurants and/or social gatherings because processed foods are often high in phosphate and other additives, which must be avoided. Constant worrying about foods leads to feelings of alienation and fears that the patient's loved ones and social circles are excluding them.
The inflexibility of recommended dietary restrictions not only decreases the quality of patients' diets and social interactions but also increases stress and anxiety. Decreasing quality of life as CKD progresses has been reported [14]. The feelings of stress, anxiety, and depression that may be the result of daily dietary self-management are potentially contributing to reduced quality of life. Many modern foods contain additives with unknown quantities of electrolytes (e.g., phosphate additives) [15,16], likely exacerbating the stress of self-management. As manufacturers are not required to list the quantity of phosphate on food labels, it is extremely challenging for patients to accurately calculate phosphate intake.
Hidden sources of dietary phosphate
Accurate quantification of dietary phosphate intake is a major challenge due to the large amount of "hidden" phosphate additives present in modern processed foods. Food-grade phosphates are widely used as preservatives and flavourings in baked goods, meats and seafood, dairy products, and beverages [17]. In baked goods such as cakes, cookies, and crackers, phosphates are used as a chemical leavening agent [17]. Sodium tripolyphosphate is most commonly used for processed meats (e.g., ham, bacon, injected poultry, chicken nuggets) to improve texture and maintain moisture [17]. Sodium phosphate is also often added to seafood during factory processing [17,18]. Tetrasodium pyrophosphate is used in processed dairy products (e.g., chocolate milk, cheese slices) to enhance texture and extend shelf life [17]. Soft drinks in particular often contain phosphoric acid as an acidifying and flavouring agent [17]. Phosphorus is ubiquitous in packaged/bottled drinks [19]. (See: Top 5 Types of Beverages Where Phosphate is Hiding). Non-carbonated and powdered beverage mixes are also likely to contain phosphate additives acting as preservatives [17]. Even worse, in foods containing phosphate additives, the proportion of digestible phosphate in total phosphate is high [20]. This suggests that the inorganic phosphate in food additives has higher bioavailability than organic phosphate from plant and animal-based foods, compounding the negative effects for patients on dialysis [20]. Results from a study by Bell et al. suggest that inorganic phosphate is more readily and completely absorbed than organic phosphate in humans [21]. Thus, patients will absorb much more phosphate when eating the processed foods with additives in comparison to natural foods, even when the total quantity of phosphate present in these foods is the same. A modern diet with a lot of hidden, inorganic phosphate additives is extremely dangerous for patients on dialysis due to the synergistic effects of increased phosphate content and almost 100% absorption of phosphate from additives.
Labels are not required to state the amount of these phosphate additives, so patients on phosphate-restricted diets are at a high risk of ingesting large amounts of "hidden" phosphates (See: Top 10 Types of Food Where Phosphate is Hiding). A study found that a diet high in processed foods increased total phosphate intake by 60% in comparison to a low-additive diet consisting mainly of fresh and minimally processed foods [22]. Leon et al. discovered that 44% of top-selling items at a grocery store contained phosphate additives. Categories of food most commonly containing phosphate additives were prepared frozen foods (72%), dry food mixes (70%), packaged meat (65%), bread and baked goods (57%), soup (54%), and yogurt (51%). The most significant sources of hidden phosphate additives were cheese (347 mg), cereal (137 mg), packaged meats (118 mg), dry food mixes (63 mg), snacks (57 mg), and condiments (53 mg) (per 100 grams of food).
Consumption of processed foods high in hidden phosphate is of particular concern during the COVID-19 pandemic. During this period, patients with CKD have found it difficult, and maybe impossible, to avoid consuming large quantities of phosphate additives. Stay-at-home orders, social distancing, and fear of going to public places such as grocery stores have made it more difficult to find fresh, healthy foods. Some patients are already living in food deserts, where getting fresh produce and quality proteins is not possible, and they have been totally cut off from even getting healthy groceries delivered due to the pandemic. These patients are forced to rely on processed foods and whatever is available in restaurants close by. Therefore, consumption of processed, shelf-stable foods containing preservatives is likely to have increased significantly.
SIDE INSERT (like a table/figure but highlighted with a different background color to draw attention to it) Top 5 Types of Beverages Where You May Not Have Known Phosphate is Hiding [23] (Figure 2).
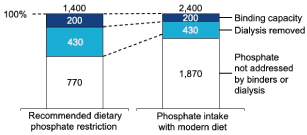
Figure 1. Average Daily Phosphate Addressed by Dialysis and Phosphate (mg/Day)
An average patient consumes ~1,400-2,400 mg of dietary phosphorus per day [21,73]. However, the average recommended daily dose of binders removes only 200-500 mg [66,68,70,71] and dialysis on average only removes 430 mg per day [2]

Figure 2. Patients on restricted diets may not realize that many popular beverages contain “hidden” phosphorus additives. A study of actual phosphorus content in powdered drinks and water enhancers found that almost 80% of the tested beverages had higher measured phosphorus than listed reference values from the Nutrition Data System for Research (NDSR) [23], indicating that patients may not have accurate information on the quantities of phosphate present in beverages and may be taking in dangerously high amounts of dietary phosphate
SIDE INSERT (like a table/figure but highlighted with a different background color to draw attention to it) Top 10 Types of Food Where You May Not Have Known Phosphate is Hiding [15,25] (Figure 3).
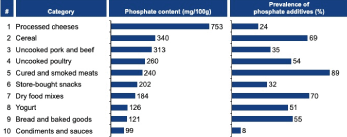
Figure 3. Hidden phosphates are present in many popular grocery store items. Although patients are likely aware that phosphate additives are abundant in processed foods, they may not suspect that uncooked proteins (e.g., poultry) are also likely to contain phosphate additives. Thus, patients would believe that seemingly “healthy” foods like chicken or yogurt are safe to eat when they are potentially sources of hidden phosphates [102]
Healthy phosphate and protein goals for patients with chronic kidney disease and end-stage renal disease
It is important for patients with CKD on dialysis to maintain normal phosphate levels so that they can avoid negative clinical outcomes and increased morbidity and mortality risks. Patients with CKD commonly experience phosphate retention resulting in elevated phosphate levels above the normal range of 2.5 to 4.5 mg/dL, due to impaired renal function as the disease progresses [25]. Phosphate retention and elevated phosphate are strongly associated with numerous negative conditions (e.g., vascular calcification, cardiovascular diseases, secondary hyperparathyroidism [26]) and increased morbidity and mortality [27]. In fact, elevated phosphate levels are by far the largest contributor to CKD mortality: 2- to 6-fold higher than other top risk factors such as hypercalcemia, hyperparathyroidism, low urea reduction ratio, and anemia (12% vs. 4%, 2%, 5%, and 6%, respectively). Abnormal phosphate levels have also been shown to be an independent risk factor for cardiovascular (CV) morbidity in patients with CKD [28,29]. There is an association between increased serum phosphate concentrations and increased risk of cardiovascular disease hospitalizations and mortality [24]. In addition to the degree of phosphate increase, time spent with elevated phosphate levels also increases the risk of CV mortality; a large multinational study of patients on dialysis found that the more time patients spent with phosphate >4.5 mg/dL over six months, the greater their risk of CV mortality. Elevated phosphate levels are directly linked to hypertension, a major cardiovascular disease risk factor that is seen in up to 90% of patients with CKD [30,31]. In addition, increased phosphate levels trigger an increase in fibroblast growth factor 23, which leads to left ventricular hypertrophy [32,33]. Therefore, reduction of serum phosphate levels through dietary restriction, dialysis, and the use of phosphate binders is a guideline-recommended, established clinical practice [34] (Table 1).
Phosphorus and protein intake correlate significantly. A major risk of dietary restrictions for patients on dialysis is protein malnutrition [35]. Patients on dialysis are recommended to consume more protein than patients with CKD that are not on dialysis [36]. Healthy individuals are recommended to maintain a protein intake of ~0.8 g/kg body weight [37]. Patients with CKD at a pre-dialysis stage are recommended to maintain a protein intake of ~0.6 g/kg body weight [38]. Patients on hemodialysis are recommended to maintain an increased protein intake of 1.2 g/kg body weight [38,39], while patients on peritoneal dialysis require even more protein (1.2 to 1.3 g/kg/day) to compensate for the loss of nutrients during treatment. Patients with ESRD have a higher resting energy expenditure than healthy individuals due to inflammation and co-morbidities such as cardiovascular disease, diabetes, and hyperparathyroidism [40-44]. Dialysis-related protein loss also contributes to the risk of insufficient energy and protein intake [45]. Protein intake <0.8 g/kg/day is associated with increased mortality [46]. A low phosphate and protein diet increases the risk of malnutrition. Protein-energy wasting (PEW) is marked by decreased body protein and fat masses [47] and is a strong risk factor for adverse outcomes and mortality in patients on dialysis [48]. Protein and energy intake, as well as overall protein-energy status, declines as CKD progresses [49], likely exacerbating the risk of PEW. Approximately 30-60% of patients with advanced CKD show evidence of PEW [50,51]. The KDOQI guidelines for nutrition in CKD recommend maintaining adequate protein and energy intake to maintain stable nutritional status, with the option of protein-energy supplementation for patients who are at risk of or experiencing protein-energy wasting [36]. Low protein intake is also a risk factor for low albumin levels [52], a strong predictor of mortality in patients on dialysis [53]. Albumin levels are an established proxy/biomarker for cardiovascular health and mortality in both patients with and without CKD [54-56]. Serum albumin levels <4.0 g/dL are associated with negative clinical outcomes in patients on dialysis [57-60]. Low albumin levels are associated with increased risk of all-cause and CV death and increases in albumin levels predict improved survival [61,62]. Factors that may lead to low albumin include inadequate protein intake [52], peritoneal membrane transport characteristics [63], and inflammation [64]. Research suggests that nutritional supplementation with protein and amino acids can increase serum albumin levels [65], thereby potentially reducing the risk of PEW and/or mortality.
Current phosphate management strategies are insufficient
Current phosphate management strategies are insufficient to achieve and maintain target phosphate levels, placing more pressure on the patients to adhere to recommended dietary restrictions. Phosphate binders, which work by binding to dietary phosphate to create insoluble complexes that are then excreted [66-70], have long been the only FDA-approved treatment indicated for hyperphosphatemia. Phosphate binders are prescribed to ~80% of US patients on dialysis. Despite the widespread use of phosphate binders, a significant proportion of patients are unable to achieve target or normal phosphate levels. Forty-two percent of patients on dialysis treated with binders had a most recent phosphate level >5.5 mg/dL and 77% had a most recent phosphate level >4.5 mg/dL. Both phosphate binders and dialysis can only remove a small portion of total dietary phosphate: hemodialysis removes ~3000 mg phosphate each week (~430 mg per day) [2], and high doses of phosphate binders can remove about 200 to 500 mg of dietary phosphate each day [68,70-72] (Figure 1). Assuming a daily phosphate intake of ~1,400 mg up to 2,400 mg [21,73], it is clear that dialysis and binders can only remove a low proportion of dietary phosphate. Sub-optimal efficacy of phosphate management strategies creates more pressure for patients to adhere to recommended dietary restrictions to avoid electrolyte overload.
Phosphate binders also change the gut microbiota by reducing bacteria living on phosphorus. Miao et al. identified seven reduced genera after the use of lanthanum carbonate for 12 weeks, demonstrating that phosphate binder use leads to decreased microbial diversity and lower network complexity [74]. Another study revealed a distinctive microbiota composition in patients receiving ferric citrate and calcium carbonate, with reduced microbial species diversity and increased microbial dysbiosis index in calcium carbonate users [75]. Further investigation on the long-term effects of different phosphate binders on the gut microflora is needed. For now, clinicians and patients should be aware of the potential for phosphate binders to damage gut flora.
Therapeutic advancements may allow for a more liberal diet
Although dietary restrictions are guideline recommended, there is very little data to support the benefits of the recommended dietary restrictions for patients on dialysis [76]. Recent therapeutic advancements that more consistently achieve normal electrolyte levels may allow patients to enjoy a more liberal diet consisting of a greater variety of healthy foods. However, foods containing preservatives and/or additives would likely never be recommended even if more effective pharmacologic therapies became available.
Therapies with novel mechanisms of action that may more effectively maintain and achieve normal electrolyte levels may allow patients to consume a more liberal diet containing healthy foods without the risk of electrolyte overload.
Several transcellular phosphate absorption inhibitors are in development and clinical trial data so far are mixed. A phase 1 trial on standardized phosphate diet of the novel drug EOS789, an inhibitor of the sodium phosphate cotransporter NaPi-2b, PiT-1, and PiT-2, showed encouraging results in patients receiving hemodialysis [77]. Nicotinamide appears to inhibit gastrointestinal NaPi2b cotransporters, thereby reducing phosphate-specific transcellular permeability [78]. However, there was a lack of significant reductions in phosphorus or FGF23 in non-dialysis CKD patients treated by lanthanum carbonate and/or nicotinamide during a 12-month trial [79]. A phase 1 study of the NaPi2b inhibitor ASP3325 showed that this therapy was not effective in reducing serum phosphorus levels in patients with ESKD [80].
One example of a novel therapy is an NHE3 inhibitor (tenapanor) that blocks phosphate absorption by conformational change(s) in claudin proteins present in tight junctions and reduces paracellular phosphate transport [81]. In contrast to phosphate binders, which do not interact with any phosphate absorption pathways, this mechanism of action targets the dominant paracellular phosphate absorption pathway and may represent a revolutionary approach to phosphate management. At 12 weeks, tenapanor administration lowered serum phosphorus in subjects from baseline concentrations of 8.1 mg/dL to 5.5 mg/dL in the efficacy analysis set [82]. In a long-term phase 3 study, at 26 weeks, tenapanor administration lowered serum phosphorus in subjects from baseline concentrations of 7.7 mg/dL to 5.1 mg/dL in the efficacy analysis set [83]. The effect of tenapanor administered in conjunction with phosphate binders has a more significant effect than binders alone: a recent trial that compared the effectiveness of a combination of tenapanor and binder vs. placebo and binder showed that tenapanor plus binder resulted in a 0.65 mg/dL larger mean serum phosphorus reduction from baseline compared to placebo plus binder [84]. The study included 236 patients undergoing maintenance dialysis with hyperphosphatemia (defined in this trial as serum phosphorus 5.5-10 mg/dL inclusive) despite receiving phosphate binder therapy (sevelamer, non-sevelamer, sevelamer plus non-sevelamer, or multiple non-sevelamer binders) [84]. Additionally, almost twice as many patients treated with tenapanor and binder achieved serum phosphorus <5.5 mg/dL compared to patients treated with placebo and binder (37-50% vs. 18-24%, p<0.05) [84]. This dual-mechanism approach may be particularly relevant for patients with persistent hyperphosphatemia [84]. In the recent OPTIMIZE TRIAL, 85.4% of patients previously treated with binders who were switched to a new treatment plan that reduced their binder dose by 50% and added tenapanor (30 mg twice daily) reported an improvement in their overall phosphorus management experience, primarily due to a better medication regimen [85,86].
Patients on dialysis are recommended to adhere to restricted diets to avoid negative consequences of electrolyte overload. While negative clinical outcomes associated with elevated phosphate levels are well-established, there is little data to support the benefits of dietary restrictions for patients on dialysis, specifically with respect to phosphorus. Furthermore, dietary restrictions decrease patients' quality of life by increasing stress, impeding social relationships, and limiting healthy food choices. Dietary restrictions have been the mainstay to management electrolytes in dialysis patients, and there has been little in the way of progress on new and novel pharmacology for CKD and dialysis patients. Recently, more inorganic phosphates which are not listed on food labels have been added to foods, making management of phosphorus extremely difficult. Now, there are new options for patients to manage their hyperphosphatemia, which may more effectively reduce and control phosphate concentrations. Incorporating these novel therapies into phosphate management strategies would allow patients to reap the benefits of healthy, liberalized diets. Broader benefits of a more liberalized diet would include improved interactions with family, a more robust social life, and less stress from the need to self-manage a restricted diet. Clinicians should consider these novel hyperphosphatemia therapies to improve both clinical outcomes and quality of life for their patients, while dietary counselling on appropriate phosphorus, sodium, potassium, fluids, and protein intake should remain a part of treatment.
Both authors made substantial contributions to the analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content. Both authors gave final approval of the version to be published and agree to act as guarantor of the work (ensuring that questions related to any part of the work are appropriately investigated and resolved).
Writing support was provided by Xelay Acumen Group, and writing support was funded by Ardelyx, Inc.
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
- Foundation NK (2019) Clinical Practice Guideline For Nutrition In Chronic Kidney Disease: 2019 Update. National Kidney Foundation.
- Hou SH, Zhao J, Ellman CF, Hu J, Griffin Z, et al. (1991) Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 18: 217-224. [Crossref]
- Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, et al. (2005) Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520-528. [Crossref]
- Faxén J, Xu H, Evans M, Jernberg T, Szummer K, et al. (2019) Potassium levels and risk of in-hospital arrhythmias and mortality in patients admitted with suspected acute coronary syndrome. Int J Cardiol 274: 52-58. [Crossref]
- Mente A, O'donnell MJ, Rangarajan S, Mcqueen MJ, Poirier P, et al. (2014) Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371: 601-611.
- Esmeray K, Dizdar OS, Erdem S, Gunal A (2018) Effect of Strict Volume Control on Renal Progression and Mortality in Non-Dialysis-Dependent Chronic Kidney Disease Patients: A Prospective Interventional Study. Med Princ Pract 27: 420-427. [Crossref]
- Fouque D, Vennegoor M, Ter Wee P, Wanner C, Basci A, et al. (2007) EBPG guideline on nutrition. Nephrol Dial Transplant 22: ii45-ii87. [Crossref]
- Lou LM, Caverni A, Gimeno JA, Moreno R, Pérez J, et al. (2012) Dietary intervention focused on phosphate intake in hemodialysis patients with hyperphosphoremia. Clin Nephrol 77: 476-483. [Crossref]
- Lynch KE, Lynch R, Curhan GC, Brunelli SM (2011) Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol 6: 620-629. [Crossref]
- Code of Federal Regulations. Title 42 Public Health. Part 494 - Conditions for Coverage of End-Stage Renal Disease Facilities. Centers for Medicare and Medicaid Services. 2016a.
- Slinin Y, Guo H, Gilbertson DT, Mau LW, Ensrud K, et al. (2011) Prehemodialysis care by dietitians and first-year mortality after initiation of hemodialysis. Am J Kidney Dis 58: 583-590. [Crossref]
- Luis D, Zlatkis K, Comenge B, García Z, Navarro JF, et al. (2016) Dietary Quality and Adherence to Dietary Recommendations in Patients Undergoing Hemodialysis. J Ren Nutr 26: 190-195. [Crossref]
- Khoueiry G, Waked A, Goldman M, El-Charabaty E, Dunne E, et al. (2011) Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J Ren Nutr 21: 438-447. [Crossref]
- Mujais SK, Story K, Brouillette J, Takano T, Soroka S, et al. (2009) Health-related quality of life in CKD Patients: correlates and evolution over time. Clin J Am Soc Nephrol 4: 1293-301. [Crossref]
- León JB, Sullivan CM, Sehgal AR (2013) The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Ren Nutr 23: 265-270. [Crossref]
- Sullivan CM, Leon JB, Sehgal AR (2007) Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr 17: 350-354. [Crossref]
- Lampila LE (2013) Applications and functions of food-grade phosphates. Ann N Y Acad Sci 1301: 37-44. [Crossref]
- Crawford DL (1980) Meat yield and shell removal functions of shrimp processing. Oregon State University Extension Marine Advisory Program.
- Moser M, White K, Henry B, Oh S, Miller ER, et al. (2015a) Phosphorus content of popular beverages. Am J Kidney Dis 65: 969-971. [Crossref]
- Karp H, Ekholm P, Kemi V, Itkonen S, Hirvonen T, et al. (2012) Differences among total and in vitro digestible phosphorus content of plant foods and beverages. J Ren Nutr 22: 416-422. [Crossref]
- Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR (1977) Physiological responses of human adults to foods containing phosphate additives. J Nutr 107: 42-50. [Crossref]
- Carrigan A, Klinger A, Choquette SS, Luzuriaga-Mcpherson A, Bell EK, et al. (2014) Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J Ren Nutr 24: 13-19. [Crossref]
- Moser M, White K, Henry B, Oh S, Miller ER, et al. (2015b) Phosphorus Content of Popular Beverages. Am J Kidney Dis 65: 969-971. [Crossref]
- Sherman RA, Ravella S, Kapoian T (2015) A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 87: 1097-1099. [Crossref]
- Craver L, Marco MP, Martínez I, Rue M, Borràs M, et al. (2007) Mineral metabolism parameters throughout chronic kidney disease stages 1-5--achievement of K/DOQI target ranges. Nephrol Dial Transplant 22: 1171-1176. [Crossref]
- Cooper K, Quarles D, Kubo Y, Tomlin H, Goodman W (2012) Relationship between reductions in parathyroid hormone and serum phosphorus during the management of secondary hyperparathyroidism with calcimimetics in hemodialysis patients. Nephron Clin Pract 121: c124-c130. [Crossref]
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG (2004) Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208-2218. [Crossref]
- Shang D, Xie Q, Ge X, Yan H, Tian J, et al. (2015) Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol 16: 107. [Crossref]
- Mcgovern AP, De Lusignan S, Van Vlymen J, Liyanage H, Tomson CR, et al. (2013) Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: a large community-based cohort study. PLoS One 8: e74996. [Crossref]
- Muntner P, Anderson A, Charleston J, Chen Z, Ford V, et al. (2010) Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 55: 441-451. [Crossref]
- Huang CX, Plantinga LC, Fink NE, Melamed ML, Coresh J, et al. (2008) Phosphate levels and blood pressure in incident hemodialysis patients: a longitudinal study. Adv Chronic Kidney Dis 15: 321-331. [Crossref]
- Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, et al. (2015) Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab 22: 1020-1032. [Crossref]
- Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, et al. (2017) FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Scientific Reports 7: 1993.
- National Kidney Foundation (2003) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1-201. [Crossref]
- Rufino M, De Bonis E, Martín M, Rebollo S, Martín B, et al. (1998) Is it possible to control hyperphosphataemia with diet, without inducing protein malnutrition? Nephrol Dial Transplant 3: 65-67. [Crossref]
- Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero J-J, et al. (2020) KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis 76: S1-S107. [Crossref]
- Joint FAO/WHO/UNU Expert Consultation On Energy And Protein, R., Food And & Agriculture Organization Of The United Nations, W. H., Organization & United Nations University 1985. Energy and protein requirements: report of a Joint FAO/WHO/UNU Expert Consultation. 1985.
- Cupisti A, Kalantar-Zadeh K (2013) Management of natural and added dietary phosphorus burden in kidney disease. Semin Nephrol 33: 180-190. [Crossref]
- Kopple JD (2001) National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 37: S66-S70. [Crossref]
- Neyra R, Chen KY, Sun M, Shyr Y, Hakim RM, et al. (2003) Increased resting energy expenditure in patients with end-stage renal disease. JPEN J Parenter Enteral Nutr 27: 36-42. [Crossref]
- Cuppari L, De Carvalho AB, Avesani CM, Kamimura MA, Dos Santos Lobão RR, et al. (2004) Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol 15: 2933-2939. [Crossref]
- Utaka S, Avesani CM, Draibe SA, Kamimura MA, Andreoni S, et al. (2005) Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr 82: 801-805. [Crossref]
- Avesani CM, Cuppari L, Silva AC, Sigulem DM, Cendoroglo M, et al., (2001) Resting energy expenditure in pre-dialysis diabetic patients. Nephrol Dial Transplant 16: 556-565. [Crossref]
- Wang AY, Sea MM, Tang N, Sanderson JE, Lui SF, et al. (2004) Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol 15: 3134-3143. [Crossref]
- Blumenkrantz MJ, Gahl GM, Kopple JD, Kamdar AV, Jones MR, et al. (1981) Protein losses during peritoneal dialysis. Kidney Int 19: 593-602. [Crossref]
- Shinaberger CS, Kilpatrick RD, Regidor DL, Mcallister CJ, Greenland S, et al. (2006) Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis 48: 37-49. [Crossref]
- Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, et al. (2008) A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391-398. [Crossref]
- Foucan L, Merault H, Velayoudom-Cephise F-L, Larifla L, Alecu C, et al. (2015) Impact of protein energy wasting status on survival among Afro-Caribbean hemodialysis patients: a 3-year prospective study. SpringerPlus 4: 452. [Crossref]
- Kopple JD, Greene T, Chumlea WC, Hollinger D, Maroni BJ, et al. (2000) Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int 57: 1688-1703. [Crossref]
- Pérez-Torres A, González Garcia ME, San José-Valiente B, Bajo Rubio MA, Celadilla Diez O, et al. (2018) Protein-energy wasting syndrome in advanced chronic kidney disease: prevalence and specific clinical characteristics. Nefrologia 38: 141-151. [Crossref]
- As'habi A, Tabibi H, Nozary-Heshmati B, Mahdavi-Mazdeh M, Hedayati M (2014) Comparison of various scoring methods for the diagnosis of protein-energy wasting in hemodialysis patients. Int Urol Nephrol 46: 999-1004. [Crossref]
- Noce A, Vidiri MF, Marrone G, Moriconi E, Bocedi A, et al. (2016) Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov 2: 16026. [Crossref]
- De Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet R, et al. (2009) Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr 19: 127-135. [Crossref]
- Hirata T, Arai Y, Yuasa S, Abe Y, Takayama M, et al. (2020) Associations of cardiovascular biomarkers and plasma albumin with exceptional survival to the highest ages. Nat Commun 11: 3820. [Crossref]
- Mehrotra R, Duong U, Jiwakanon S, Kovesdy CP, Moran J, et al. (2011) Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis 58: 418-428. [Crossref]
- Herrmann FR, Safran C, Levkoff SE, Minaker KL (1992) Serum Albumin Level on Admission as a Predictor of Death, Length of Stay, and Readmission. Arch Intern Med 152: 125-130. [Crossref]
- Amaral S, Hwang W, Fivush B, Neu A, Frankenfield D, et al., (2008) Serum albumin level and risk for mortality and hospitalization in adolescents on hemodialysis. Clin J Am Soc Nephrol 3: 759-767. [Crossref]
- Goldwasser P, Mittman N, Antignani A, Burrell D, Michel MA, et al. (1993) Predictors of mortality in hemodialysis patients. J Am Soc Nephrol 3: 1613-1622. [Crossref]
- Owen WF, Lew NL, Liu Y, Lowrie EG, Lazarus JM (1993) The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001-1006. [Crossref]
- Phillips A, Shaper AG, Whincup PH (1989) Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet 2: 1434-1436. [Crossref]
- Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, Mcallister CJ, Alcorn H, et al. (2005b) Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 20: 1880-1888. [Crossref]
- Msaad R, Essadik R, Mohtadi K, Meftah H, Lebrazi H, et al. (2019) Predictors of mortality in hemodialysis patients. Pan Afr Med J 33: 61. [Crossref]
- Han DS, Lee SW, Kang SW, Choi KH, Lee HY, et al. (1996) Factors affecting low values of serum albumin in CAPD patients. Adv Perit Dial 12: 288-292. [Crossref]
- Kaysen GA, Dubin JA, Müller HG, Rosales L, Levin NW, et al. (2004) Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int 65: 1408-1415. [Crossref]
- Kalantar-Zadeh K, Braglia A, Chow J, Kwon O, Kuwae N, et al. (2005a) An anti-inflammatory and antioxidant nutritional supplement for hypoalbuminemic hemodialysis patients: a pilot/feasibility study. J Ren Nutr 15: 318-331. [Crossref]
- PhosLo® gelcaps (calcium acetate): 667 mg [prescribing information]. Waltham, MA: Fresenius Medical Care North America. 2011.
- VELPHORO® (sucroferric oxyhydroxide) [prescribing information]. Waltham, MA: Fresenius Medical Care North America. 2013.
- FOSRENOL® (lanthanum carbonate) [prescribing information]. Lexington, MA: Shire US Inc. 2004.
- AURYXIA® (ferric citrate) tablets [prescribing information]. Cambridge, MA: Keryx Biopharmaceuticals Inc. 2017a.
- RENVELA® (sevelamer carbonate) [prescribing information]. Cambridge, MA: Genzyme Corp. 2000.
- Daugirdas JT, Finn WF, Emmett M, Chertow GM (2011) The phosphate binder equivalent dose. Semin Dial 24: 41-49. [Crossref]
- Kidney Disease: Improving Global Outcomes (2011) KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 7: 1-59. [Crossref]
- Mcclure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ (2017) Dietary Sources of Phosphorus among Adults in the United States: Results from NHANES 2001-2014. Nutrients 9: 95. [Crossref]
- Miao YY, Xu CM, Xia M, Zhu HQ, Chen YQ (2018) Relationship between Gut Microbiota and Phosphorus Metabolism in Hemodialysis Patients: A Preliminary Exploration. Chin Med J (Engl) 131: 2792-2799. [Crossref]
- Wu PH, Liu PY, Chiu YW, Hung WC, Lin YT, et al. (2020) Comparative Gut Microbiome Differences between Ferric Citrate and Calcium Carbonate Phosphate Binders in Patients with End-Stage Kidney Disease. Microorganisms 8: 2040. [Crossref]
- Kalantar-Zadeh K, Tortorici AR, Chen JL, Kamgar M, Lau WL, et al. (2015) Dietary restrictions in dialysis patients: is there anything left to eat? Semin Dial 28: 159-68. [Crossref]
- Hill Gallant KM, Stremke ER, Trevino LL, Moorthi RN, Doshi S, et al. (2021) EOS789, a broad-spectrum inhibitor of phosphate transport, is safe with an indication of efficacy in a phase 1b randomized crossover trial in hemodialysis patients. Kidney Int 99: 1225-1233. [Crossref]
- Eto N, Miyata Y, Ohno H, Yamashita T (2005) Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant 20: 1378-1384. [Crossref]
- Ix JH, Isakova T, Larive B, Raphael KL, Raj DS, et al. (2019) Effects of Nicotinamide and Lanthanum Carbonate on Serum Phosphate and Fibroblast Growth Factor-23 in CKD: The COMBINE Trial. J Am Soc Nephrol 30: 1096-1108. [Crossref]
- Larsson TE, Kameoka C, Nakajo I, Taniuchi Y, Yoshida S, et al. (2018) NPT-IIb Inhibition Does Not Improve Hyperphosphatemia in CKD. Kidney Int Rep 3: 73-80. [Crossref]
- King AJ, Siegel M, He Y, Nie B, Wang J, et al. (2018) Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 10: eaam6474. [Crossref]
- Block GA, Rosenbaum DP, Yan A, Chertow GM (2019) Efficacy and Safety of Tenapanor in Patients with Hyperphosphatemia Receiving Maintenance Hemodialysis: A Randomized Phase 3 Trial. J Am Soc Nephrol 30: 641-652. [Crossref]
- Chertow GM, Yang Y, Rosenbaum DP (2020) Long-term safety and efficacy of tenapanor for the control of serum phosphorus in patients with chronic kidney disease on dialysis. American Society of Nephrology (ASN) Kidney Week 2020.
- Pergola PE, Rosenbaum DP, Yang Y, Chertow GM (2021) A Randomized Trial of Tenapanor and Phosphate Binders as a Dual-Mechanism Treatment for Hyperphosphatemia in Patients on Maintenance Dialysis (AMPLIFY). J Am Soc Nephrol 32: 1465-1473. [Crossref]
- Sonani B, Naganathan S, Al-Dhahir M (2021) Hypernatremia. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Patient-Reported Experience with Tenapanor in the OPTIMIZE Trial. Kidney Week 2021.
- Shrimanker I, Bhattarai S (2020) Electrolytes. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Bai W, Li J, Liu J (2016) Serum phosphorus, cardiovascular and all-cause mortality in the general population: a meta-analysis. Clin Chim Acta 461: 76-82. [Crossref]
- Lopes MB, Karaboyas A, Bieber B, Pisoni RL, Walpen S, et al. (2020) Impact of longer-term phosphorus control on cardiovascular mortality in hemodialysis patients using an area under the curve approach: results from the DOPPS. Nephrol Dial Transplant 35: 1794-1801. [Crossref]
- Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'agostino RB, et al. (2007) Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879-885. [Crossref]
- Zhang D, Bi X, Liu Y, Huang Y, Xiong J, et al. (2017) High Phosphate-Induced Calcification of Vascular Smooth Muscle Cells is Associated with the TLR4/NF-κb Signaling Pathway. Kidney Blood Press Res 42: 1205-1215. [Crossref]
- Mendes M, Resende L, Teixeira A, Correia J, Silva G (2017) Blood pressure and phosphate level in diabetic and non-diabetic kidney disease: Results of the cross-sectional "Low Clearance Consultation" study. Porto Biomed J 2: 301-305. [Crossref]
- Heaney RP, Dowell MS, Hale CA, Bendich A (2003) Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22: 142-146. [Crossref]
- Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, et al., (1998) High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9: 1845-1852. [Crossref]
- Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, et al. (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393-4408. [Crossref]
- Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, et al. (2014) Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349-360. [Crossref]
- Cheng SP, Liu CL, Liu TP, Hsu YC, Lee JJ (2014) Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm 2014: 709024. [Crossref]
- Osto E, Fallo F, Pelizzo MR, Maddalozzo A, Sorgato N, et al. (2012) Coronary Microvascular Dysfunction Induced by Primary Hyperparathyroidism is Restored After Parathyroidectomy. Circulation 126: 1031-1039. [Crossref]
- Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, et al. (2009) Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76: 422-427. [Crossref]
- Fluid Overload in a Dialysis Patient [Online]. National Kidney Foundation. 2016b.
- Claure-Del Granado R, Mehta RL (2016) Fluid overload in the ICU: evaluation and management. BMC Nephrol 17: 109. [Crossref]
- Sherman RA, Mehta O (2009) Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis. Clin J Am Soc Nephrol 4: 1370-1373. [Crossref]



