Schwannoma is a rare, benign, slow-growing neoplasm which arises from the peripheral nerve sheaths. Vagal nerve schwannoma is often asymptomatic and without neurological deficit. Correct diagnosis is challenging since the tumor can mimic a malignant lateral neck mass. Magnetic resonance imaging is the accepted golden standard for preoperative localization and characterization of the tumor. In cases with benign, small, slow-growing tumors where significant morbidity is expected after surgery, conservative management might be more beneficial. Otherwise, the management is complete surgical excision.
We present a case of a 71-year old woman with a left-sided neck mass accompanied by mild hoarseness. On clinical examination, we found a 3.5×3×2 cm slightly mobile, non-tender mass at the left level II. A malignant metastasis of the lymph node was suspected, and the patient underwent complete excision of the tumor. Histological examination revealed evidence of vagus schwannoma. Follow-up several months after surgical removal showed no evidence of recurrence or any new onset long-term side-effects.
schwannoma, vagal nerve tumor, vocal fold
The diagnosis of a lateral neck mass can be challenging and often malignancy is suspected, especially in elderly patients. Patients presenting with a lateral neck mass are currently evaluated using a standard guideline including ultrasound of the neck, fine needle aspiration biopsy (FNAB) and computer tomography and/or magnetic resonance imaging (MRI). Even with these modalities the diagnosis can be uncertain [1-5].
Schwannoma is a benign mass of neurogenic origin. This slow-growing neoplasm arises from schwann cells that produce nerve sheaths of peripheral, autonomic and cranial nerves. Schwannomas make up 5% of all benign soft-tissue tumors [6]. Of these, around 45% are located in the head or neck region [7]. Growth rates are variable but may grow 2.5 to 3 mm a year. It is very rare, that they undergo malignant transformation [8].
Vagal schwannomas are often asymptomatic, but the patients most frequently present with a slow-growing neck swelling and hoarseness. A pathognomonic clinical finding is coughing when the mass is palpated due to stimulation of the vagal nerve. It most frequently occurs in the third and fourth decade and female/male ratio is 1.5 [8].
Surgical resection is the first choice of treatment, but conservative management and debulking surgery may be considered in some cases. Up to two thirds preferred using the technique of intracapsular enucleation, whereas extracapsular resection accounted for one third. Transcervical incision was used in most (93.2%) cases [8].
A 71-year old woman was referred to our clinic of otorhinolaryngology with a left-sided mass which had gradually progressed in size over more than a year. The patient was treated by her general practitioner with a loop diuretic, a calcium channel blocker, a beta blocker and an angiotensin-converting-enzyme inhibitor in combination with a thiazide to treat hypertension and congestive heart failure (New York Heart Association class II). She had no history of compression symptoms, pain or dysphagia. She had hoarseness and rapid onset of vocal fatigue. She did not have any other local complaints. During the last months she had increasingly shortness of breath on exertion. Clinical examination revealed a 3.5×3×2 cm firm, slightly mobile and non-tender mass in the left level II. Furthermore, a mild goitre was recognized.
Nasal fiberoptic endoscopy showed left-sided vocal fold paralysis. Chest X-ray revealed enlarged heart size, signs of congestive heart failure and suspicion of pericardial effusion. Subsequently, PET-CT showed no evidence of pericardial effusion. The patient was sufficiently treated by her general practitioner with diuretics and antihypertensive drugs.
For further characterization of the mass, a contrast-enhanced CT scan was made, which showed a well-circumscribed, oval, hypodense mass with a few calcifications in an irregular border located in level II of the left neck (Figures 1A and 1B). The mass displaced the internal jugular vein anterolaterally and the common carotid artery posteromedially. There was no abnormal cervical lymph node enlargement. The transverse size was 32×37 mm and the craniocaudal dimension was 50 mm.
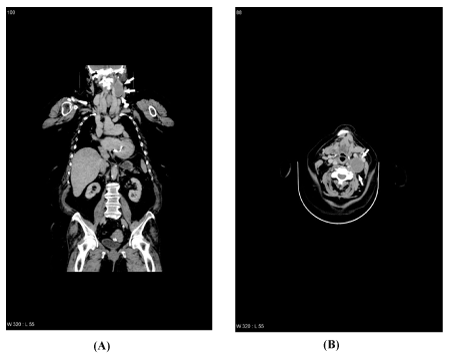
Figure 1. Contrast-enhanced CT scan (coronal and transversal view) of the neck showing the left-sided vagal schwannoma (white arrows)
PET-CT showed mild increased F-18 fluorodeoxyglucose (FDG) uptake in the palatine tonsils, interpreted as a normal metabolic phenomenon, and in the circumference of the lesion in the left side of the neck. There was also increased inhomogeneous FDG-uptake in a multinodular goitre. Significantly increased FDG-uptake in the sigmoid colon was detected, and the patient was referred to relevant further investigation. There was no sign of neurofibromatosis. The radiological evaluation suggested a cystic mass with some metabolism at the borders either malignant or benign.
Neither chest X-ray nor PET-CT was suspicious of any primary tumor since the uptake in the tonsils was deemed non-pathologic by the radiologist. Preoperative ultrasonography demonstrated a mass located to level III and IV of the left neck measuring 6×4×3 cm, closely related to adjacent nerves. Initial FNAB was inconclusive for diagnostics. The second FNAB was also non-diagnostic.
The patient underwent surgical excision of the tumor. Intraoperatively, the tumor was identified medial to the jugular vein and was closely attached to nerve fibers (Figures 2A and 2B). When stimulated with a nerve stimulator (Aesculap, Aesculap Inc., USA), bradycardia was induced. Two adjacent lymph nodes were removed in toto with no histopatological sign of malignancy. The nerve was preserved during surgery. Perioperative histopathology of the primary mass suggested schwannoma, thus no further surgical procedures were carried out (i.e. tonsillectomy or random biopsies).
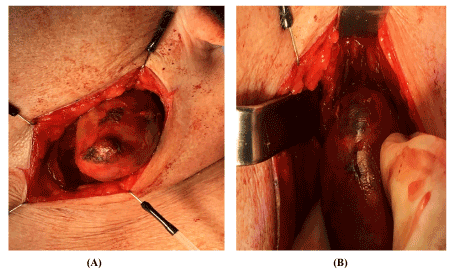
Figure 2. Intra-operative pictures of the schwannoma.
Subsequently, the patient was excluded from the fast-track programme for suspected cancer. After successful resection, there was no evidence of further vagal, accessory or marginal mandibular nerve lesions. Nasal fiberoptic endoscopy showed the well-known left-sided vocal fold paralysis. The postoperative period was uneventful and the patient was discharged on the second postoperative day.
Final histopathology showed a benign schwannoma with cystic degeneration and areas of both Anthony A and Anthony B patterns. There was no sign of malignancy. S-100 staining was positive. Excised lymph nodes showed no sign of malignancy.
Postoperatively, the patient was referred to vocal fold training. Protection of the healing scar from the sun was advised.
At clinical follow up 14 months after surgery, stroboscopic laryngoscopy showed decreased alignment of the vocal folds due to the left-sided palsy, however well compensated. The hoarseness resolved, and the patient experienced improved quality of voice. There was no need for further vocal fold training. There was no recurrence of the schwannoma.
Schwannomas, also known as neuromas or neurilemomas, are benign tumors arising from schwann cells of peripheral, cranial and autonomic nerve sheaths. They comprise 5% of all benign soft tissue tumors [6]. It is estimated, that one third are located in the head and neck region [9]. The parapharyngeal space is the most common site of non-vestibular schwannomas located in the head and neck region [10,11]. In one study, a total of 36 neoplasms of the vagal nerve were reviewed [12]. Of these, 50% were paragangliomas, 31% were schwannomas, 14% were neurofibromas, and 6% neurofibrosarcomas.
Based on a review of 22 studies presenting a total of 53 patients with cervical vagal schwannomas, the female/male ratio was 1.5 and the onset of diagnosis was reported most frequently in the third and fourth decade. Median diameter was 5 cm (ranging from 2 to 10 cm). The literature supports that most cervical schwannomas (64%) are left-sided [13].
The clinical presentation depends on the anatomical location of the tumor; however, the most frequent symptom is hoarseness [8]. Pain or cough may also occur, which may cause suspicion of malignant disease. Preoperatively, vocal fold paralysis was found in around 12% of cases [14]. In one study, nearly one third of subjects (31.1%) with vocal fold paralysis had a tumor, of which laryngeal cancer was the most common cause, followed by thyroid and lung neoplasms, respectively [15].
Most patients present with a slow-growing, often asymptomatic mass in the neck. Neurological deficit are rare. A pathognomonic finding is paroxysmal cough while palpating the tumor. On palpation, the tumor is usually movable horizontally but not vertically.
Our patient was a 71-year old woman who experienced a slow-growing mass in the left side of the neck and hoarseness. Initially, the tumor was interpreted as a parathyroid cyst. However, due to the age of the patient and clinical findings, a lymph node metastasis could not be ruled out, for which reason the patient entered the fast-track programme for detection of malignancy. The dyspnoea on exertion is most likely due to chronic heart failure, as MRI did not show any displacement of the trachea or other possible causes.
FNAB can be used to differentiate benign from malignant tumors, but FNAB has a rather low specificity when evaluating lateral neck masses [16]. In our case, FNAB was inconclusive twice. Cytological diagnosis of schwannomas was in two studies obtained in only 20% and 12.5% of cases, respectively [13,17]. Therefore, some authors do not recommend FNAB. However, FNAB can be of great value by suggesting a diagnosis or rule out important differential diagnosis like lymph node metastasis. Most authors do not recommend core biopsy [14]. In Denmark, a core biopsy is a relative contraindication in suspected metastasis without capsular penetration.
MRI is the golden standard for preoperative diagnostic imaging and planning of surgical removal. Schwannomas appear with low signal intensity on T1-weighted imaging and with high signal intensity on T2-weighted imaging [18]. Noncontrast-enhanced CT scan can be made, but a contrast-enhanced CT scan is to be advised in order to assess the vascularity of the tumor and adjacent tissue. Ultrasonographical characteristics include oval, solitary, well-defined, hypoechoic masses with posterior acustic enhancement and absence of a hilum as seen in lymph nodes [19]. Due to uncharacteristic history, clinical examination, radiological findings and low rate of cytological diagnosis when FNAB is made, preoperative diagnosis of schwannomas is challenging. Furthermore, FNAB of schwannoma is usually painful to the patient.
In our case, with the benefit of hindsight an MRI would have been of greater value in order to detect low stage of laryngeal cancer and to evaluate the extent and relationship to adjacent tissue of a potential schwannoma. In cases like this, where malignancy cannot be excluded, tumor management depends on an overall assessment based on tumor size, rate of growth, patient symptoms, co-morbidities and remaining life expectancy. This case was an elderly woman with a growing neck mass accompanied by hoarseness. According to present guidelines [20], a PET-CT is the golden standard in investigating a suspected cancer with unknown primary origin.
There are many differential diagnoses to neck tumors and patients with vagal schwannoma have neurological deficits extremely rarely. Consequently, an accurate preoperative diagnosis is difficult. Below, we summarize the most relevant differential diagnoses.
Inflammatory adenopathy of viral aetiology is the most common cause of cervical lymphadenopathy. It arises typically during upper respiratory infections and resolves within one to two weeks. The lymph nodes are usually tender and mobile and are located in the submandibular region or along with the jugular chain. They are usually less than 1 cm on the shortest diameter.
Branchial cleft cyst is a congenital defect that most often is recognized in late childhood or early adulthood when the cyst is infected. The most common type is the second branchial cleft cyst that is usually located anterior to the sternocleidomastoid muscle and inferior to the angle of the mandible. The cyst is usually painless but can become tender and swollen during upper respiratory tract infections.
Metastatic lymphadenopathies must be ruled out, especially in the case of an adult over 40 years of age with a persistent (>3 weeks), unexplained neck mass. Metastatic lymph nodes are often asymptomatic. Possible symptoms are related to the site of the primary tumor, including oral swelling, unexplained persistent sore throat, oral ulcer, hoarseness, odynophagia and dysphagia [3]. Metastatic lymph nodes are usually single-sided, but 10% are located bilaterally in the neck [21].
Paragangliomas are rare, slow-growing, vascularized neuroendocrine tumors. Some secrete catecholamines. Parasympathetic paragangliomas are non-functional and almost always in the skull base and neck. In carotid body tumors, the patient typically presents with a painless neck mass. Jugulotympanic paragangliomas can present with a pulsatile tinnitus with or without conductive hearing loss. Catecholamine-secreting tumors can lead to episodic palpitations, sweating, headache, flushing, hypertension, pallor and anxiety. Diagnosis is based on measuring catecholamines and metanephrines in the blood and/or urine followed by imaging. Non-secretory skull base and neck paragangliomas can be diagnosed with ultrasound, CT or MRI.
Lipomas are benign, slow-growing, soft, usually asymptomatic masses that develop superficially in the subcutaneous layer of the skin.
Neurofibromatosis type 1 and 2 (NF1 and NF2) must be suspected when multiple schwannomas are present. Other clinical signs of NF1 include café-au-lait macules, freckles in the axillary and/or inguinal region and Lisch nodules. Clinical manifestations of the rarer NF2 are bilateral vestibular schwannomas, schwannomas arising from other cranial nerves, meningiomas, neuropathies and spinal tumors.
Schwannomatosis (the third type of NF) must be considered once NF1 and NF2 have been excluded. It is a rare disorder suspected in individuals with multiple schwannomas arising from cranial or peripheral nerves, except the presence of bilateral vestibular schwannomas.
Laryngeal pouches (laryngocele), diverticula and other embryonic malformations can also give rise to various swellings on the neck [22].
Two histological patterns are significantly suggestive of schwannomas; Antoni A and Antoni B regions. The cellular architecture of Antoni A is characterized by densely arranged and elongated nuclei called palisades, creating clear, waving nucleus-free areas called Verocay bodies. Schwann cells express the S-100 protein, especially in Antoni A regions. Antoni B regions are characterized by more loosely arranged cells and fibers [23].
The treatment is complete surgical excision with preservation of the nerve when possible. Most studies advocate intracapsular excision in order to minimize the risk of nerve injury [13,24]. Subtotal resection must be considered when tumor is too large and total resection implies a significant risk of nerve damaging, especially in elderly or patients with comorbidity where general anesthesia is poorly tolerated. Recurrence is extremely rare. In cases where malignancy is suspected, more extended excisions might be necessary with the risk of permanently damaging the vagal nerve. In these cases, end-to-end anastomosis or the use of nerve graft should be considered. Some surgeons argue that schwannoma resection in early stages must be avoided, unless the tumor causes significant morbidity, because of the risks of long-term neurological deficit as Horner’s syndrome, dysphagia and vocal fold paralysis.
Radiosurgery as an alternative to surgical resection is worth considering for high-risk patients and patients with residual and recurrent tumors. Studies have reported good tumor control and lower morbidity following stereotactic radiosurgery of non-vestibular cranial nerve schwannomas. However, the studies did not include neck schwannomas as in this case. Randomized clinical trials are needed to increase the ability to compare the microsurgical and radiosurgical techniques [25,26].
The most frequent postoperative complication is vocal fold paresis. Despite a stroboscopic finding at follow-up of left-sided vocal fold paralysis and associated incomplete closure of vocal fold, our patient experienced a substantial improvement of voice quality, presumably due to contralateral vocal fold compensation. An objective clinical evaluation of the voice showed no dysphonia. Other less frequent complications include coughing, dysphagia, secretion, facial nerve palsy and Horner’s syndrome [8]. Preoperatively, the patient should be informed about such potential complications.
In conclusion, cervical vagal nerve schwannomas are uncommon benign tumors, which extremely rarely undergo malignant transformation. The majority of patients present with a slow-growing single-sided neck swelling with no neurological deficit. The definitive treatment is total surgical excision of tumor with preservation of the nerve to prevent recurrence and maintain nerve function. Hoarseness is a common complication following surgery, but in most cases, it recovers spontaneously and can be aided by voice therapy. Awareness of schwannoma as a possible differential diagnosis to lateral neck masses medial to the sternocleidomastoid muscle is important in order to plan adequate imaging and make an individualized decision based on a reflected balance between benefits and risks of surgery.
Competing interests
The authors declare that they have no conflict of interest.
Author contributions
HE: Has contributed to the design of the case report, drafted the manuscript, found references, been in contact with collaborators and approved the final manuscript. ERR: Has treated the patient. Has contributed to the design of the case report, drafted the manuscript, found references, and been in contact with the patient and relatives. She has approved the final manuscript. LKS: Has treated the patient. Has revised the manuscript critically for intellectual content, has contributed substantially to the interpretation of the patient data and approved the final manuscript.
- Dimitrijevic MV, Jesic SD, Mikic AA, Arsovic NA, Tomanovic NR (2010) Parapharyngeal space tumors: 61 case reviews. Int J Oral Maxillofac Surg 39: 983-989. [Crossref]
- Khafif A, Segev Y, Kaplan DM, Gil Z, Fliss DM (2005) Surgical management of parapharyngeal space tumors: a 10-year review. Otolaryngol Head Neck Surg 132: 401-406. [Crossref]
- Tikka T, Pracy P, Paleri V (2016) Refining the head and neck cancer referral guidelines: a two-centre analysis of 4715 referrals. Br J Oral Maxillofac Surg 54: 141-150. [Crossref]
- Bhattacharyya N (1999) Predictive factors for neoplasia and malignancy in a neck mass. Arch Otolaryngol Head Neck Surg 125: 303-307. [Crossref]
- Schwetschenau E, Kelley DJ (2002) The adult neck mass. Am Fam Physician 66: 831-838. [Crossref]
- Biswas D, Marnane CN, Mal R, Baldwin D (2007) Extracranial head and neck schwannomas--a 10-year review. Auris Nasus Larynx 34: 353-359. [Crossref]
- Das Gupta TK, Brasfield RD, Strong EW, Hajdu SI (1969) Benign solitary Schwannomas (neurilemomas). Cancer 24: 355-366. [Crossref]
- Cavallaro G, Pattaro G, Iorio O, Avallone M, Silecchia G (2015) A literature review on surgery for cervical vagal schwannomas. World J Surg Oncol 13: 130. [Crossref]
- Chiun KC, Tang IP, Prepageran N, Jayalakshmi P (2012) An extensive cervical vagal nerve schwannoma: a case report. Med J Malaysia 67: 342-344. [Crossref]
- Malone JP, Lee WJ, Levin RJ (2005) Clinical characteristics and treatment outcome for nonvestibular schwannomas of the head and neck. Am J Otolaryngol 26: 108-112. [Crossref]
- Leu YS, Chang KC (2002) Extracranial head and neck schwannomas: a review of 8 years experience. Acta Otolaryngol 122: 435-437. [Crossref]
- Green JD Jr, Olsen KD, DeSanto LW, Scheithauer BW (1988) Neoplasms of the vagus nerve. Laryngoscope 98: 648-654. [Crossref]
- Kang GC, Soo KC, Lim DT (2007) Extracranial non-vestibular head and neck schwannomas: a ten-year experience. Ann Acad Med Singapore 36: 233-238. [Crossref]
- Chiofalo MG, Longo F, Marone U, Franco R, Petrillo A, et al. (2009) Cervical vagal schwannoma. A case report. Acta Otorhinolaryngol Ital 29: 33-35. [Crossref]
- Seyed Toutounchi SJ, Eydi M, Golzari SE, Ghaffari MR, Parvizian N (2014) Vocal cord paralysis and its etiologies: a prospective study. J cardiovasc Thorac Res 6: 47-50. [Crossref]
- Grønlund S, Mey K, Andersen E, Rasmussen ER (2016) The true malignancy rate in 135 patients with preoperative diagnosis of a lateral neck cyst. Laryngoscope Investig Otolaryngol 1: 78-82. [Crossref]
- Yu GH, Sack MJ, Baloch Z, Gupta PK (1999) Difficulties in the fine needle aspiration (FNA) diagnosis of schwannoma. Cytopathology 10: 186-194. [Crossref]
- Lin J, Martel W (2001) Cross-sectional imaging of peripheral nerve sheath tumours: characteristics signs on CT, MR imaging, and sonography. AJR Am J Roentgenol 176: 75-82. [Crossref]
- King AD, Ahuja AT, King W, Metreweli C (1997) Sonography of peripheral nerve tumors of the neck. AJR Am J Roentgenol 169: 1695-1698. [Crossref]
- The Danish Head and Neck Cancer Study Group (DAHANCA) guidelines. https://www.dahanca.oncology.dk/Brows_Web_Guidelines
- Grau C, Johansen LV, Jakobsen J, Geertsen P, Andersen E, et al. (2000) Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish society for head and neck oncology. Radiother Oncol 55: 121-129. [Crossref]
- Ward PH, Fredrickson JM, Strandjord NM, Valvassori GE (1963) Laryngeal and pharyngeal pouches. Surgical approach and the use of cinefluorographic and other radiologic techniques as diagnostic aids. Laryngoscope 73: 564-582. [Crossref]
- Wippold FJ II, Lubner M, Perrin RJ, Lämmie M, Perry A (2007) Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. AJNR Am J Neuroradiol 28: 1633-1638. [Crossref]
- Torossian JM, Beziat JL, Abou Chebel N, Devouassoux-Shisheboran M, Fischer G (1999) Extracranial cephalic schwannomas: a series of 15 patients. J Craniofac Surg 10: 389-394. [Crossref]
- Hasegawa T (2013) Stereotactic radiosurgery for nonvestibular schwannomas. Neurosurg Clin N Am 24: 531-542. [Crossref]
- Zabel A, Debus J, Thilmann C, Schlegel W, Wannenmacher M (2001) Management of benign cranial nonacoustic schwannomas by fractionated stereotactic radiotherapy. Int J Cancer 96: 356-362. [Crossref]


