Background and objectives: Stroke in tuberculous meningitis (TBM) has been reported in approximately 13-57% of patients with TBM. There are several mechanisms postulated for stroke in tuberculous meningitis including the role of various cytokines, chemokines and vascular endothelial growth factor (VEGF). VEGF is a potent regulator of endothelial permeability. There is a paucity of data regarding comparison of VEGF levels in patients of TBM with and without stroke. The aim of the study was to compare Serum VEGF in patients of tuberculous meningitis with and without Stroke and correlate it with various clinical, biochemical and radiological features.
Methods: We compared 40 patients of probable or definite TBM with 40 age and sex matched patients of TBM with stroke.The two groups were compared for duration of illness, BMRC staging, various clinical parameters, CSF parameters (cytology, glucose and protein),MRI Brain scan features and serum VEGF levels.
Results: The serum VEGF levels were significantly higher in TBM patients with stroke as compared to patients without stroke. The mean serum VEGF levels were 499.338 ± 230.320 pg/ml in patients of TBM without stroke and 1218.372 ± 570.114 pg/ml in patients of TBM with stroke. On multivariate analysis, out of the significantly different variables between patients of TBM without stroke and with stroke(i.e. duration of illness at presentation, hemiparesis, GCS score, Stage 3 BMRC, CSF protein concentration, CSF sugar, CSF lymphocyte count, presence of hydrocephalus on MRI Brain scan and serum VEGF) only GCS score and serum VEGF levels were significant.
Interpretation and conclusion: The stroke in tuberculous meningitis was correlated to higher serum VEGF levels, lower GCS score. We conclude that features such as presentation in stage 3 BMRC, poor GCS at presentation, increased duration of illness at presentation, hydrocephalus on MRI and high serum VEGF levels may predict the occurrence of stroke in TBM.
VGEF, TBM, STROKE
BMRC - British Medical Research Council; CSF - Cerebro-Spinal Fluid; cells/μl – cells/microliter; ELISA - Enzyme Linked Immunosorbent Assay; GCS - Glasgow Coma Score; HIV - Human Immuno Deficiency Virus; mg/dl- milligram/deciliter; MRI – Magnetic Resonance Imaging; pg/ml- picogram/milliliter; TBM - Tuberculous Meningitis; VEGF – Vascular Endothelial Growth Factor
Globally approximately 10 million people developed tuberculosis (TB) causing approximately 1.3 million deaths among HIV negative patients and 300,000 deaths in HIV positive patients in 2008 [1]. Central nervous system (CNS) involvement is one of the most devastating clinical manifestations of tuberculosis (TB). It constitutes approximately 11% of extrapulmonary TB [2].
Tuberculous Meningitis is the most destructive type of tuberculous meningitis as it is associated with high morbidity and mortality. Stroke is a common complication of tuberculous meningitis. Recent studies has reported stroke in approximately 25% of patients with TBM, however older studies have shown the incidence to be as high as 57% [3-5].The mortality rates of 13-30% in patients of TBM, while older studies have shown mortality to be as high as 60%. Mortality in TBM patients increases with the occurrence of stroke [6-8]. There are few studies which have described clinical features, CSF findings and radiological features as predictors of morbidity and mortality in TBM but prediction of stroke in TBM is difficult because of unclear underlying pathological mechanisms and variations in host immunity [9].
Cerebrovascular complications of tuberculous meningitis occurring typically as multiple or bilateral lesions in the territories of the middle cerebral artery perforating vessels are termed as tuberculous vasculopathy. The proposed pathogenesis is direct invasion, proliferation of the endothelium and necrosis of the blood vessel wall leading to thrombosis of the blood vessel. There is some evidence that vasospasm may mediate strokes early in the course of the disease and proliferative intimal disease can lead to stroke later in the disease course [10].
There are several mechanisms postulated for the occurrence of stroke in TBM including the role of various cytokines and chemokines including VEGF [11-13]. The VEGF (Vascular Endothelial Growth Factor) is a potent regulator of endothelial permeability. VEGF has been related to disruption of blood brain barrier and cerebral edema in brain tumor, bacterial meningitis, and acute ischemic stroke [13,14]. There is conflicting data on the serum VEGF levels and the occurrence of stroke in patients of TBM. If some serum or CSF marker can predict, which patients of TBM will develop stroke in early stages, then anti-platelet therapy such as aspirin and dipyridamole can be started in early stages of TBM, which may reduce and prevent occurrence of stroke in patients with TBM. The aim of the study was to compare Serum VEGF in patients with tuberculous meningitis with and without Stroke and correlate it with various clinical, biochemical and radiological features
Study design
40 Patients aged 14-60 years of probable or definite TBM and 40 age and sex matched patients of TBM with clinical stroke were enrolled in the study and formed two groups [15] (Figure 1). The patients were taken from out patient department and in patient department at University College of Medical Sciences and GTB Hospital, Delhi from November 2015 to April 2017. Ethical clearance was taken from the Institutional Ethical Committee at University College of Medical Sciences. Informed consent was taken from the patients before being included in the study. The patients who were unable to give consent (Glasgow score <15), it was taken from the family members of the patient. Suspected patient of meningitis not fulfilling the criteria for probable or definite TBM, patients who had once or more times taken treatment for tuberculosis in the past and patients with any chronic systemic disease like DM, HT or chronic inflammatory disease or autoimmune disorder or immunodeficiency or malignancy were excluded from the study.
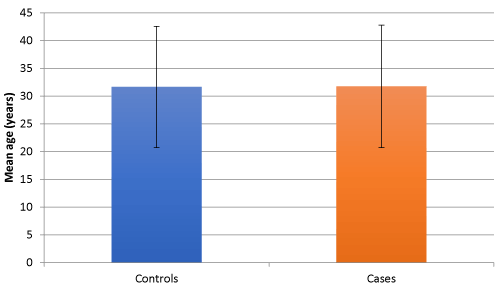
Figure 1. Mean age of two study groups
The CSF was collected and separated in two vials one plain and one EDTA-vial. Plain vial was sent for CSF sugar and protein. EDTA-vial sample was immediately sent for cytology at the pathology department at University College of Medical Sciences and GTB Hospital, 5ml plain vial sample was made to clot and then centrifuged to separate serum and stored at -200C, for estimation of serum VEGF levels Serum VEGF level estimated was done by human VEGF-A Elisa Kit by Diclone SAS, France at Biochemistry Department at University College of Medical Sciences and GTB Hospital. Brain MRI was done using 3 Tesla machine T1, T2, fluid attenuated inversion recovery (FLAIR), diffusion weighted imaging (DW1), and T1 contrast images were obtained and the presence of exudates, hydrocephalus, tuberculoma and infarction was noted. Data was entered into Microsoft Excel and after cleaning was analyzed using SPSS software v 20.0. Distributed variables were summarized as mean and SD. Both the groups with normal distribution curve were compared to each other using unpaired Student's t-test for quantitative variables and chi-square/fisher-exact test for qualitative variables and were categorized as significant or insignificant keeping the p-value <0.05. Groups with unknown distribution were compared by Man Whitney U test. Multiple logistic regression was done using SPSS software. The two groups were compared for various clinical parameters, biochemical parameters (CSF cytology, glucose and protein), MRI Brain features and serum VEGF levels.
Comparing 40 age and sex matched subjects of tuberculous meningitis with the evidence of stroke in MRI Brain (cases) with 40 subjects of tuberculous meningitis without evidence of stroke (controls), we found that the mean duration of illness in cases at presentation was significantly higher (33.10 ± 9.345 days) as compared to controls (24.60 ± 6.920 days) (p value of < 0.01). There was no statistically significant difference for the presence of clinical symptoms such as, history of fever, headache, vomiting or seizures (Figure 2). The presence of nuchal rigidity also, was not significantly different between the cases and controls. Median GCS score in controls was 13 (2 to 14) and in cases was 11(9 to 13), and the difference was statistically significant (p = 0.025). 20 cases presented in BMRC stage 3 as compared to control group, in which only 9 cases were in BMRC stage 3 and the difference was statistically significant (p value 0.011). The number of patients in BMRC stage 1 and 2 was 17 and 9 in control group and 10 and 10 in study group respectively, this difference was not statistically significant (p value 0329 for stage 1 BMRC and 0.098 for stage 2 BMRC). CSF protein concentration was significantly higher in cases as compared to controls with a p value of <0.001. CSF sugar was significantly lower in cases as compared to controls with a p value of 0.039. CSF total leucocyte count was significantly higher in cases as compared to controls with a p value of 0.024. CSF lymphocyte counts were significantly higher in cases as compared to controls with a p value of 0.18 (Figure 3). There was no significant difference in CSF polymorphonuclear cells between cases and controls. Statistically higher presence of Hydrocephalus in MRI was found in cases as compared to controls, with a p value of 0.019. Presence of tuberculomas on MRI was a statistically insignificant different between cases and controls (p = 0.644). Basal meningeal enhancement was present in all patients in controls and cases groups, as it was a defining criteria for tuberculous meningitis. Serum VEGF levels were significantly higher in cases (1218.372 ± 570.114 pg/ml) as compared to controls (499.338 ± 230.320 pg/ml) with a p value of <0.01.
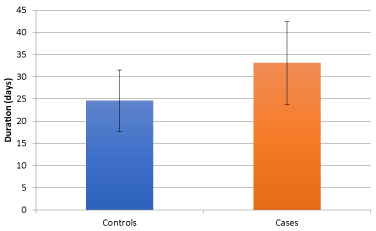
Figure 2. Mean duration of illness of two study groups
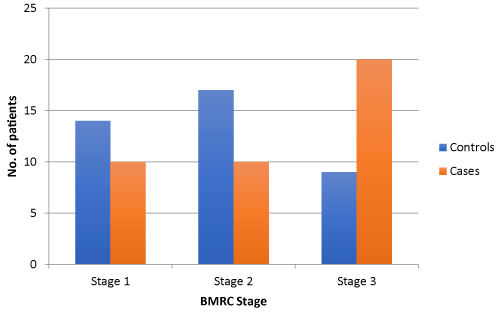
Figure 3. Distribution of patients according to BMRC stage of two study groups
To find the best correlating factor to stroke in TBM, we performed multiple logistic regression analysis. On applying stepwise multiple logistic regression analysis we found that out of the significantly different variables between patients of TBM without stroke and with stroke, found on direct comparison (i.e. duration of illness at presentation, hemiparesis, GCS score, Stage 3 BMRC, CSF protein concentration, CSF sugar, CSF lymphocyte count, presence of hydrocephalus on MRI Brain scan and serum VEGF) only GCS score and serum VEGF levels were significant. Thus, stroke in tuberculous meningitis was correlated to higher serum VEGF levels, lower GCS score (Figure 4).
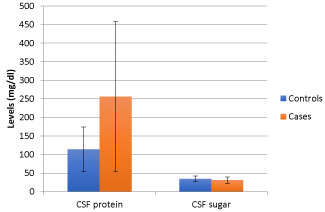
Figure 4. CSFprotein and sugar characteristics in the two study groups
1. Comparison of serum VEGF levels between patients of TBM with stroke as compared to those without stroke and it’s correlation with stroke in tuberculous meningitis
In our study the mean serum VEGF levels were 499.338 ± 230.320 pg/ml in patients of TBM without stroke and 1218.372 ± 570.114 pg/ml in patients of TBM with stroke (Table 1). Similar serum VEGF levels have been reported by Nuzhat H, et al. where they quantified VEGF levels by enzyme linked immunoassay (ELISA) in serum and cerebrospinal fluid in 20 cases each with active and inactive TBM as well as 22 cases of intra parenchymal tuberculoma.Mean serum VEGF levels in active TBM, inactive TBM and tuberculoma was 694.93 ± 820.66 pg/ml, 499.6 ± 238.33 pg/ml and 541.0 ± 389.0 pg/ml, respectively. Significantly increased VEGF levels in CSF were found in active TBM cases (106.0 ± 50.0 pg/ml) compared to inactive TBM cases (14.7 ± 10.0 pg/ml, p < 0.001). Immunohistochemical staining of excised tuberculoma demonstrated high expression of VEGF. Serial decrease in serum VEGF levels was observed with increasing duration of therapy in tuberculoma. They concluded that increased CSF and serum VEGF levels are a measure of activity of the disease in neurotuberculosis and its gradual decrease over a period of time is probably an indicator of therapeutic response [13]. In our study the levels of Serum VEGF were significantly higher in patients of TBM with stroke as compared to TBM without stroke with a p value of <0.001. The levels of serum VEGF were 499.338 ± 230.320 pg/ml in patients of TBM without stroke and 1218.372 ± 570.114 pg/ml in patients of TBM with stroke (Table 1).
Table 1. VEGF levels in study group and control group
Variable |
Control |
Cases |
P value |
Significance |
Serum VEGF (pg/ml) |
499.338 ± 230.320 |
1218.372 ± 570.114 |
<0.01 |
Significant |
On multiple logistic regression analysis between patients of tuberculous meningitis with stroke and those without stroke serum VEGF levels and poor GCS score correlated with stroke in tuberculous meningitis.Only a couple of studies are present in literature comparing serum VEGF levels in patients of TBM with stroke and those without stroke.In the study done by Misra UK, et al. in TBM patients, evaluating the levels of serum VEGF in TBM and in TBM with stroke, 40 patients of TBM were evaluated, it was seen that serum VEGF levels in TBM patients (100.7 ± 110.6 pg/ml) was higher compared to healthy controls (60.6 ± 20.3 pg/ml). The serum VEGF levels were also higher in patients of TBM with MRI evidence of infarction (131.4 ± 150.7 pg/ml) as compared to those without infarction (73.0 ± 41.4 pg/ml) though it didn’t reach significance [16]. In the second study conducted by van Der flier, et al., blood and CSF samples of 26 children of tuberculous meningitis were compared with 20 children of fever in whom meningitis was ruled out by lumbar puncture. VEGF was detectable in the CSF of 15 of 26 (58%) of the TB meningitis patients (98 ± 31 pg/mL) and in none of the control patients (all < 25 pg/mL; P = 0.039). The plasma VEGF concentrations were also significantly higher (p value 0.045) in TBM patients (182 ± 52 pg/ml) as compared to controls (69 ± 12 pg/ml). The calculated VEGF index was 486 ± 976, a finding that indicated intrathecal production of VEGF. In their study cranial CT scan demonstrated periventricular edema in 66 % patients, tuberculoma in 17 % and cerebral infarction in 38 %, but the presence of these pathologic findings on cranial CT had no relation to CSF VEGF concentrations [17].
2. Correlation of various clinical features, CSF characteristics and neuroimaging features as predictors of stroke in patients of TBM
In our study, the mean age of TBM patients was 31.65 ± 10.93 in control group and 31.75 ± 9.35 in study group, which shows that TBM patients were young and actively working individuals highlighting the socioeconomic burden of TBM in such individuals, which is known to cause high mortality and loss of disability adjusted life years. Similar observations have been made by other studies which have shown that TBM usually affects young and active individuals [18,19]. Out of 80 patients included in the study nearly an equal number of males and females were distributed among cases and controls.
In our study, the two groups didn’t differ statistically as per the presenting symptoms (fever, headache, vomiting, weight loss and seizures) and signs (nuchal rigidity and cranial nerve palsy) are concerned except for duration of illness at presentation, hemiparesis at presentation, GCS score at presentation and the presence of stage 3 BMRC. TBM patients with stroke were more likely to present with poor GCS and in advanced stage (stage 3 BMRC) of TBM and exhibited hemiparesis at presentation (p = 0.05) (Table 2-4).On analysis of the CSF characteristics between the two groups CSF protein, total leucocyte count and lymphocytic count was significantly higher in TBM patients with infarct as compared to TBM patients without infarct and CSF sugar was significantly lower in TBM with infarct as compared to TBM without infarct. The Neuroimaging finding of Hydrocephalus was significantly associated with stroke in TBM and the finding of tuberculoma had no significant correlation with stroke in TBM (Figure 5).
Table 2. Demographic and clinical profile of two study groups
|
|
|
P value |
|
-
|
31.65 10.930 |
31.75 11.031 |
-
|
Non significant |
|
|
-
|
-
|
-
|
Non significant |
|
-
|
-
|
Duration of illness (days) |
24.60 6.927 |
-
|
|
|
|
-
|
-
|
-
|
Non significant |
|
-
|
-
|
-
|
Non significant |
|
-
|
-
|
-
|
Non significant |
Weight loss |
-
|
-
|
-
|
Non significant |
|
-
|
-
|
-
|
Non significant |
Nuchal rigidity |
-
|
-
|
-
|
Non significant |
C. N palsy |
-
|
-
|
-
|
Non significant |
|
-
|
-
|
-
|
|
GCS score |
-
|
-
|
-
|
|
Stage 1 BMRC |
-
|
-
|
-
|
Non significant |
Stage 2 BMRC |
-
|
-
|
-
|
Non significant |
Stage 3 BMRC |
-
|
-
|
-
|
|
Table 3. CSF characteristics in the two study groups
Variable |
Controls |
Cases |
P value |
Significance |
CSF protein(mg/dl) |
113.73 ± 60.232 |
256.15 ± 202.44 |
<0.001 |
Significant |
CSF sugar(mg/dl) |
34.63 ± 7.791 |
30.78 ± 8.628 |
0.039 |
Significant |
CSF TLC (cells/μl) |
145 ± 79.93 |
198.95 ± 123.481 |
0.024 |
Significant |
CSF DLC |
Lymphocytes |
73.73 ± 7.851 |
78.40 ± 9.339 |
0.018 |
Significant |
Polymorphonuclear cells |
26.03 ± 7.823 |
22.60 ± 9.652 |
0.085 |
Non significant |
Table 4. MRI findings in the two groups
Variable |
Controls |
Cases |
P value |
Significance |
Tuberculomas |
16 |
14 |
0.644 |
Non significant |
Hydrocephalus |
9 |
19 |
0.019 |
Significant |
Basal meningeal enhancement |
40 |
40 |
-* Defining criteria for tuberculous meningitis |
Infarct |
0 |
40 |
<0.001 |
Significant |
-*statistics were not calculated, as SPSS software considered it as a constant as it was present in all cases and controls.
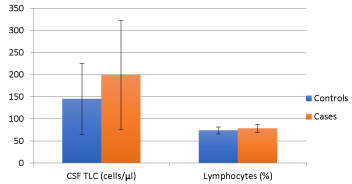
Figure 5. CSFTLC and lymphocytecharacteristics in the two study groups
In a study done by Anuradha H K, et al. on univariate analysis predictors of stroke in TBM were age >25 years, cranial nerve involvement (focal neurological deficit) and presence of basal exudates or meningeal enhancement. Poor visual acuity and stage III BMRC of tuberculous meningitis were other significant predictors of stroke. In our study basal meningeal enhancement or exudates were not compared between two groups as it was the inclusion criteria of TBM. In concordance with our study stroke in TBM correlated to stage III BMRC of tuberculous meningitis in the above study (Figure 6). But in contrast to our study other variables such as low Glasgow coma scale score, hydrocephalous, CSF parameters and presence of tuberculoma were not significantly related to the occurrence of stroke in the above study [19].
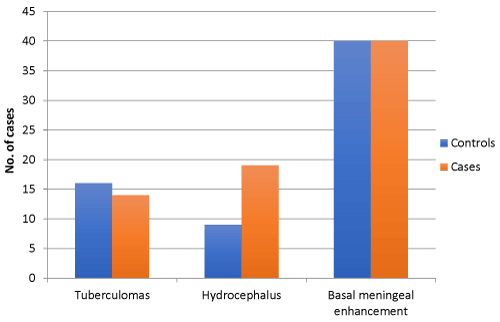
Figure 6. MRI findings in the two study groups
In another study done by Misra UK, et al. stroke in TBM was significantly related to the presence of hydrocephalus, basal exudate and hypertension and it also correlated to factors such as age, sex, level of consciousness, diabetes, HIV status, CSF protein, CSF leucocytes, CSF sugar and presence of tuberculoma on MRI Brain. In our study presence of hypertension was an exclusion criterion and basal meningeal enhancement or exudates were not compared between two groups as it was the inclusion criteria of TBM. In concurrence to our study stroke in TBM correlated to presence of hydrocephalus. But in discordance to our study other variables such as CSF protein, CSF leucocytes, and CSF sugar were not related to stroke in TBM in the above study [18] (Figure 7).
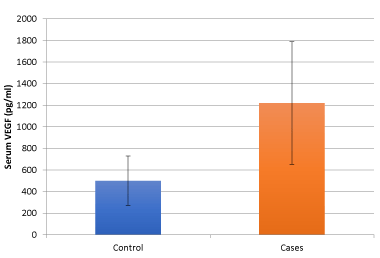
Figure 7. VEGF levels in study group and control group
In contrast to our results and above findings, a study which compared demographic features, clinical, laboratory, and neuroradiological findings between patients of TBM with stroke and those without stroke found that the percentage of cerebrospinal fluid neutrophils was significantly higher in patients with stroke than in patients without stroke. There were no significant differences between the groups with respect to other clinical and laboratory features, including demographic features and cerebrospinal fluid findings [20]. In our study hemiparesis at presentation was significantly associated with stroke in tuberculous meningitis (p = 0.05), corroborating with similar observation made in another study, where they found that appearance of focal neurological deficit at presentation was associated with occurrence of stroke in tuberculous meningitis [21]. We conclude that features such as presentation in stage 3 BMRC, poor GCS at presentation, increased duration of illness at presentation, hydrocephalus on MRI and high serum VEGF levels may predict the occurrence of stroke in TBM, but further prospective studies are needed to delineate the role of above parameters in predicting stroke. If any such parameter or marker of disease can be developed which can predict the occurrence of stroke in early stages of TBM, then anti-platelet therapy such as aspirin and dipyridamole can be started in the early stages of TBM, which may reduce and prevent the occurrence of stroke in patients with TBM.
- Global Tuberculosis Report (2018) World Health Organization.
- Ohene SA, Bakker MI, Ojo J, Toonstra A, Awudi D, et al. (2019) Extra-pulmonary tuberculosis: A retrospective study of patients in Accra, Ghana.PLoS One14: e0209650. [Crossref]
- Azeemuddin M, Alvi A, Sayani R, Khan MK, Farooq S, et al. (2019) Neuroimaging Findings in Tuberculosis: A Single-Center Experience in 559 Cases. JNeuroimaging.
- Wasay M, Farooq S, Khowaja ZA, Bawa ZA, Ali SM, et al. (2014) Cerebral infarction and tuberculoma in central nervous system tuberculosis: frequency and prognostic implications. J Neurol Neurosurg Psychiatry 85: 1260-1264. [Crossref]
- Shukla R, Abbas A, Kumar P, Gupta RK, Jha S, et al. (2008) Evaluation of cerebral infarction in tuberculous meningitis by diffusion weighted imaging. J Infect 57: 298-306. [Crossref]
- Farinha NJ1, Razali KA, Holzel H, Morgan G, Novelli VM (2000) Tuberculosis of the central nervous system in children: a 20-year survey.J Infect41: 61-68. [Crossref]
- Soria J1,2, Metcalf T3,2, Mori N4, et al. (2019) Mortality in hospitalized patients with tuberculous meningitis.BMC Infect Dis19: 9. [Crossref]
- Verdon R, Chevret S, Laissy J-P, Wolff M (1996) Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis 22: 982-988. [Crossref]
- Misra UK1, Kalita J, Roy AK, Mandal SK, Srivastava M (2000) Role of clinical, radiological, and neurophysiological changes in predicting the outcome of tuberculous meningitis: a multivariable analysis.J Neurol Neurosurg Psychiatry68: 300-303. [Crossref]
- Lammie GA, Hewlett RH, Schoeman JF, Donald PR (2009) Tuberculous cerebrovascular disease: a review. J Infect 59: 156-166. [Crossref]
- Misra UK1, Kalita J, Srivastava R, Nair PP, Mishra MK, et al. (2010) A study of cytokines in tuberculous meningitis: clinical and MRI correlation.Neurosci Lett483: 6-10. [Crossref]
- Matsuyama W1, Hashiguchi T, Umehara F, Matsuura E, Kawabata M, et al. (2001) Expression of vascular endothelial growth factor in tuberculous meningitis.J Neurol Sci186: 75-79. [Crossref]
- Husain N1, Awasthi S, Haris M, Gupta RK, Husain M (2008) Vascular endothelial growth factor as a marker of disease activity in neurotuberculosis.J Infect56: 114-119. [Crossref]
- Huang Z, Cheng L, Guryanova OA, Wu Q, Bao S (2010) Cancer stem cells in glioblastoma—molecular signaling and therapeutic targeting. Protein Cell 1: 638-655. [Crossref]
- Marias S, Thwaites G, Shoeman JF (2010) Tuberculous meningitis: a uniform clinical case definition. Lacet Infect Dis 10: 803-812. [Crossref]
- Misra UK, Kalita J, Singh AP, Prasad S (2013) Vascular endothelial growth factor in tuberculous meningitis. Int J Neurosci 123: 128-132. [Crossref]
- van der Flier M, Hoppenreijs S, van Rensburg AJ, Ruyken M, Kolk AHJ, et al. (2004) Vascular endothelial growth factor and blood-brain barrier disruption in tuberculous meningitis. Pediatr Infect Dis J 23: 608-613. [Crossref]
- Kalita J, Misra UK, Nair PP (2009) Predictors of stroke and its significance in the outcome of tuberculous meningitis. J Stroke Cerebrovasc Dis 18: 251-258. [Crossref]
- Anuradha HK, Garg RK, Agarwal A, Sinha MK, Verma R, et al. (2010) Predictors of stroke in patients of tuberculous meningitis and its effect on the outcome.QJM103: 671-678. [Crossref]
- Koh SB, Kim BJ, Park MH, Yu SW, Park KW, et al. (2007) Clinical and laboratory characteristics of cerebral infarction in tuberculous meningitis: a comparative study. J ClinNeurosci 14: 1073-1077. [Crossref]
- Kalita J, Misra UK, Ranjan P (2007) Predictors of long-term neurological sequelae of tuberculous meningitis: a multivariate analysis. Eur J Neurol 14: 33-37. [Crossref]







