Yaws is a destructive soft-tissue infection caused by the bacterium Treponema pallidum pertenue that has remained on the World Health Organization's agenda for eradication for more than half a century. Affecting predominantly young children living in warm, humid settings, the recurrence of yaws is a result of a stall in worldwide surveillance, continuing to earn its place on the list of neglected tropical diseases. This lack of monitoring now limits our understanding of its prevalence. The combination of disease latency and poor recognition may lead to unnecessary morbidity. A renewed campaign against this devastating, yet treatable disease has offered new hope. The purpose of this review is to increase the awareness of the condition and discuss the new approach amongst the global health community.
Yaws, neglected tropical diseases, treponema, eradication
Yaws is an endemic relapsing treponematosis caused by Treponema pallidum subspecies pertenue “and is one of 17” neglected tropical diseases affecting primarily young children (<15 years of age) living in tropical regions with an average temperature of 80°F [1-3]. In endemic regions, it is known by many names, including pian (French); framboesia (German, Dutch); buba (Spanish); bouba (Portuguese).The disease has been targeted for eradication since the 1950s by the World Health Organization (WHO). This period marked a once promising progression towards this goal from 1952 to 1964, when the Global Yaws Control Program successfully treated more than 300 million people and reduced prevalence by 95% in 46 endemic countries. The global effort was derailed when control programs were discontinued in many countries and reporting diminished. Subsequently, awareness of the global disease burden diminished to the point where isolated endemic pockets have re-emerged, therefore limiting current estimates of disease prevalence. Recognizing and formally addressing this trend in 2012, the WHO released a proposal for global eradication by 2020 via intensified neglected tropical disease management [4].
The diagnosis of yaws is predominantly made by recognition of the clinical features. While not typically fatal, it can cause severe physical disfiguration in up to 10% of cases. Similar to other treponemes, yaws is capable of a broad range of clinical manifestations and has multiple stages of progression, which makes for a challenging diagnosis. A high index of suspicion is required in young children from endemic regions. Early recognition and initiation of antibiotics is curative. A feasible treatment emerged from a study in 2012 published in The Lancet, demonstrating that the use of a single dose of oral azithromycin offers similar efficacy to intramuscular penicillin injection and is easier to administer [5]. This approach transcends previous barriers, such as the necessity for skilled medical workers, refrigeration and administration of painful injections [6]. In order to promote a successful campaign, the global health community should be cognizant of the various clinical manifestations, improve recognition and focus on more accurate reporting and treatment.
Reports of yaws were described in 1807 by William Mariner as a disease affecting the arms, legs, and perineal regions of children living in the South Pacific [7]. Thomas Sydenham once described the disease as "familial syphilis," suggesting easy transmissibility in close quarters. In 1949, the World Health Assembly recognized yaws as a part of the endemic treponematoses. Intramuscular (IM) penicillin and benzathine benzylpenicillin have been shown to be effective in lowering the prevalence of clinically active disease [8,9], but follow-up studies have reported that active yaws emerged in participants previously believed to be disease-free, suggesting an infectious, asymptomatic phase [9]. This observation also suggested the need to treat asymptomatic household contacts [9].
The World Health Organization estimated a global prevalence of 2.5 million cases in the 1990s. Since then, the WHO estimates that 450,000 new cases have emerged, a majority of which arose in central and West Africa (400,000). Recent numbers have been difficult to obtain because of inconsistent monitoring. In 2010, results from clinical and serological surveys using the rapid plasma reagin (RPR) test showed that not only has the disease persisted in some areas but now appears to be attenuated. Latent cases with less overt clinical features are leading to a gross underestimation of the true number of affected individuals [10]. It is well known that clinical diagnosis is deceptively challenging; therefore a heightened sense of urgency to eradicate the disease is warranted to avert further spread [11,12].
Eradication of yaws has already been declared in India. The WHO saw the number of cases decline from 3571 in 1996 to 735 in 1997 and eventually zero in 2004 [13]. Yaws was officially eliminated in India in 2006 [14]. According to the WHO, Ecuador also reported interruption of disease transmission in 2003, however this is pending confirmation. The key elements in achieving this goal include a steadfast political will, proper recognition and clinical suspicion and adequate funding, all of which are necessary moving forward.
The Third WHO Consultation on Yaws Eradication in March 2014 in Geneva, Switzerland provided the most recent update on the campaigns in Congo, Ghana, Papua New Guinea and Vanuatu. The approach uses the Morges strategy [15,16] for antibiotic administration with 6-month follow-up surveys to detect and treat remaining cases. The search for a rapid diagnostic test using the dual path platform (DPP) syphilis assay has been studied and compared to the RPR and venereal disease research laboratory (VDRL) test in an effort to create an efficacious point-of-care confirmation of disease. This would avoid local dependence on laboratory services in high prevalence areas that are equally likely to be the most destitute. There have been drastic reductions in the numbers of yaws cases when an adequate number of the community is treated (greater than 90% of an endemic community) using the total community treatment policy [16]. The number of new cases declining by 90% in just six months of treatment in Lihir, Papua New Guinea [17]; however, quoting funding concerns, and officials remain cautiously optimistic. It is estimated that pharmaceutical companies would need to donate 200 million tablets (or 92 million grams) to treat approximately 40 million people at risk from 2015 until 2020, with the possibility of Pfizer and other companies providing the medications free of charge [17]. Aside from medications, concerns still remain regarding resource mobilization for the remaining campaigns. It is estimated that ancillary services and testing could cost as little as $100 - 200 million [17] up to $1 billion in support to treat the remaining 12 endemic countries. The next countries queued for mass treatment includes the Solomon Islands, Cameroon and Indonesia. Post-treatment surveillance aims to be more efficient using rapid dual point-of-care testing, however future updates will shed better light on the current approach.
Yaws is spread through direct skin-to-skin, nonsexual contact with exudative skin lesions [2,3,18,19]. Open lesions may harbor the infectious spirochete for up to six months and spontaneously involute. Variable relapse can occur after latent periods, allowing further transmission. In endemic regions with oscillating wet and dry seasons, the clinical manifestations and prevalence of infectious lesions are higher during the rainy season. Compared to syphilis, yaws does not cross the placenta and therefore is not transmitted in utero, nor does it penetrate the central nervous system [2,20].
Clinical manifestations of yaws are separated into clinically active and inactive forms. Clinically active lesions are subdivided into infectious and non-infectious. Yaws follows a similar clinical course as untreated syphilis: primary lesions, secondary lesions, and then tertiary lesions occur if the infection progresses (Table 1).
Table 1. Clinical classification of Active versus Inactive yaws
Clinically Active |
Lesion |
Infectiousness* |
Clinically Inactive |
Lesion |
Infectious
|
Papilloma |
+++ |
Late |
Gummata |
Papillomata |
+++ |
|
Ulcers |
Maculopapules |
++ |
|
Gangosa** |
Papules |
++ |
|
Sabre tibia° |
Micropapules |
++ |
|
|
Macules |
+ |
|
|
Nodules |
+ |
|
|
Plaques |
+ |
|
|
Non-infectious
|
Hyperkeratosis |
|
|
|
Bone & Joint lesions |
|
|
|
* +++ highly infectious
|
++ very infectious
|
+ infectious
|
|
|
**Ulceration of the nasal septum and palate [10]
°"Curved" tibia secondary to chronic osteitis [10] |
Table adapted from: Handbook on Endemic Treponematoses. Geneva, 1984:11. http://whqlibdoc.who.int/ publications/1984/9241541768.pdf.
Primary lesions appear 9 to 90 days after initial exposure 2. The lesions are known as "mother yaws" and are often pruritic, facilitating further spread of sores via autoinoculation by scratching, spreading the bacteria from the non-indurated primary papule to uninfected skin 1-3,18,19. Primary lesions can persist for 3 to 6 months and spontaneously involute with scarring. Lesions range from ulcers to granulomatous papules () and are easily confused with venereal syphilis if the genitalia are involved 1,2,19,21. Further confounding rapid recognition of primary lesions is the climate-dependent variation in appearance. Macule-predominant lesions may occur during the dry season Papillomata may occur in the axilla and anal folds (Figure 4).Multiple, expanding, annular lesions beginning as solitary erythematous papules have also been described, with spread to the face, upper trunk and palmoplantar areas. Hypopigmented erythematous papules may also recur at sites of prior involuted lesions.
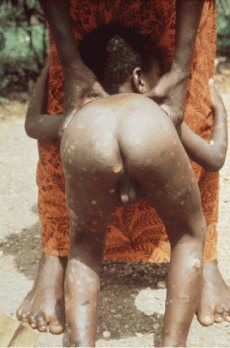
Figure 1. Early, diffuse cutaneous macular and ulcerative lesions extending into the anal cleft. Photo credit: CDC/Dr. Peter Perine
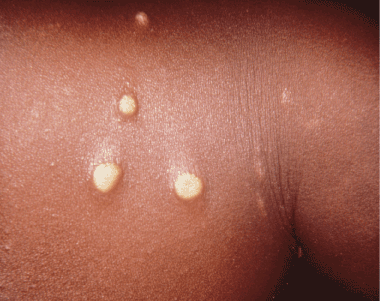
Figure 2. Early papillomata that had been present for 2 weeks, contracted by a mother who had been breastfeeding her yaws infected child. Photo credit: CDC/Dr. Peter Perine
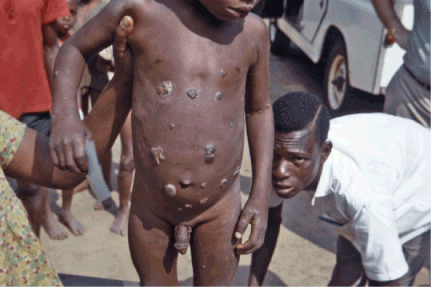
Figure 3. Multiple ulcerative skin lesions. Photo credit: CDC/Dr. Lyle Conrad
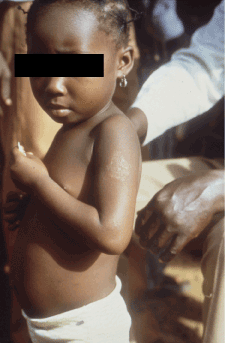
Figure 4. Early squamous macule. Photo credit: CDC/Dr. Peter Perine
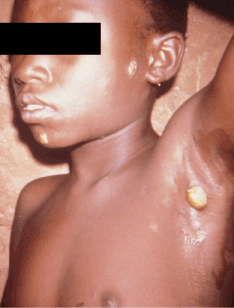
Figure 5. Early papilloma indicative of primary yaws affecting the axilla. Photo credit: CDC/Dr. Peter Perine
Secondary lesions arise near the primary site, causing widespread "daughter yaws," which are generally non-destructive 9. Latency periods are variable but it has been observed that secondary lesions can emerge up to 20 years after infection 9 and are often associated with constitutional symptoms and lymphadenopathy 1. Both primary and secondary stages may occur simultaneously and involute with or without scarring 22
Tertiary lesions, or "late yaws", are associated with soft tissue destruction in 10% of cases 2 leading to deep ulceration, gangosa (destruction of the maxilla), hyperkeratotic palmar and plantar lesions, and bony involvement These lesions typically present 5 years following initial presentation of early yaws. While this stage is not infectious, the clinical appearance indicates threatening phases of deep tissue damage, leading to severe, irreversible disability in up to 10 - 20% of untreated individuals 2,18.
Yaws periostitis causes severe bone pain and swelling. Early periostitis does not pose a major threat to morbidity if treated promptly. The condition should be high on the differential diagnosis for bone pain in young children from endemic regions. The most common finding in a series of seven case reports in Papua New Guinea was hypertrophic periostitis of long bones 23. Osteoperiostitis (Figures 6 and 10) has distinct features compared to syphilis. In yaws, the distribution of articular involvement is often bilateral, including the radius, ulna and phalanges. Bony involvement in syphilis is pauciostotic, often sparing the hands and feet 23.
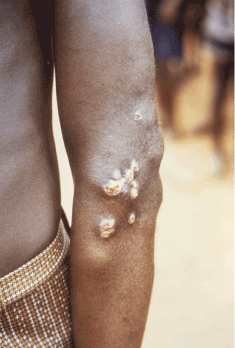
Figure 6. Non-infectious lesion on the elbow. Photo credit: CDC/Dr. Peter Perine
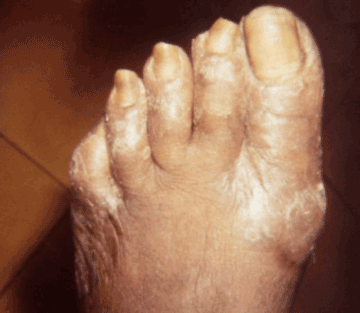
Figure 7. Late yaws of the foot, causing ambulatory dysfunction due to soft tissue masses, referred to as “crab yaws”. Photo credit: CDC/Dr. Susan Lindsley
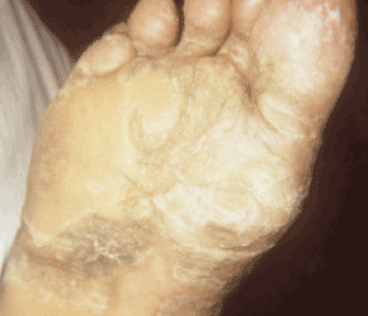
Figure 8. Late yaws of the foot. Photo credit: CDC/Dr. Susan Lindsley

Figure 9. Confluent papillomata with ulcerations on the shin. These lesions were present for several years and are consistent with mixed early and late lesions. Photo credit: CDC/Dr. Peter Perine

Figure 10. Tibial “sabre deformity” is consistent with late tertiary yaws infection. This can also be observed in younger patients with congenital syphilis as well as older patients with Yaws. Photo credit: CDC/Dr. Peter Perine

Figure 11. Late tertiary yaws affecting the skull. This is the result of untreated yaws, with destructive effects occurring at least five years after the initial infection. Photo credit: CDC/Dr. Peter Perine
By histologic comparison, early infectious yaws is epidermotropic, whereas syphilis (primary or secondary) is mesodermotropic, preferring the deeper layers of the mesoderm. The primary histopathologic difference between yaws and syphilis is that yaws is typically observed in the epidermis without affecting perivasculature [22], whereas syphilis penetrates deeper towards the dermal-epidermal junction [23]. Furthermore, yaws is not associated with any neurological nor cardiovascular involvement [2].
Yaws is diagnosed clinically, with laboratory confirmation of disease [22]. Serologic evaluation can be used for confirmation of the diagnosis however cannot differentiate between syphilis and yaws subspecies [24]. Dark field microscopy can be used for detection but also cannot differentiate between the two. The available testing includes the RPR, fluorescent treponemal antibody absorption (FTA-ABS) test, VDRL test and T. pallidum hemagglutination assay (TPPA). Currently, the search for a sensitive and specific rapid diagnostic test that allows accurate point-of-care results is underway for diagnostic confirmation [25]. A community surveillance study compared the ChemBio DPP syphilis screen and confirm test against the gold standard serology tests for non-treponemal detection (RPR) and treponemal detection (TPPA). The sensitivity and specificity of the RDT against TPPA was 58.5% and 97.6%, respectively. The sensitivity and specificity of the RDT against RPR was 41.7% and 95.2%, respectively [26]. The sensitivity of the ChemBio DPP syphilis screen was correlated to higher RPR titers, having a sensitivity of 92.0% for an RPR titer of >1:16. This study showed a lower sensitivity and specificity compared to the only prior evaluation of this assay [27], suggesting that the test is most useful for confirmation in patients with highly active clinical disease [26]. This rapid screening test does not perform as well in patients with latent disease nor useful in areas with low prevalence [26]. The search for a cost-effective rapid screening test in asymptomatic patients remains to be seen, however, does serve a useful function in post-mass drug administration monitoring.
Penicillin had historically been the drug of choice for treponemal infections. Recent advances have determined that a single oral dose of azithromycin (30 mg/kg) is non-inferior to intramuscular benzathine benzylpenicillin (50,000 units/kg) in children ages 6 months to 15 years of age [28]. Availability in the areas that need it is a problem however. Azithromycin is available in limited supply for the pilot projects and the pharmaceutical industry only supplies azithromycin for elimination of blinding trachoma as part of the International Trachoma Initiative, although arrangements for donation by Pfizer are in imminent.
Oral azithromycin is preferable over IM benzathine penicillin for logistical reasons. Administration of the oral antibiotic spares children from painful injections and the need for refrigeration [29,30]. The recommended dosage of azithromycin is 30 mg/kg (maximum dose 2 grams) administered as a single dose (Table 2). Syrup formulations are available for children younger than 6 years old. If syrup is unavailable, then a tablet can be pulverized and dissolved in water.
Table 2. Dosing chart for yaws treatment by age
Dosage schedule with oral Azithromycin for treatment of Yaws by age |
Age (years) |
Total Dose (mg) |
# of tablets |
Syrup (mL) |
<6 |
500 |
1 |
12.5 |
6 to 9 |
1000 |
2 |
|
10 to 15 |
1500 |
3 |
|
> 15 |
2000 |
4 |
|
*Adapted from the World Health Organization Summary Report on the Eradication of Yaws,
http://apps.who.int/iris/bitstream/10665/75528/1/WHO_HTM_NTD_IDM_2012.2_eng.pdf
Positive outcomes correlate directly with earlier onset of treatment. Treatment of early lesions with a single dose of penicillin reduces infectivity within 24 hours, with complete healing within two weeks [31]. Skin lesions may require several months to heal. Therefore, a strict follow-up regimen is warranted to detect latent cases and to prevent the late stages and severe soft tissue and bony deficits [18]. On a global scale, similar results from mass azithromycin treatment have demonstrated a significant impact. Previous predictors of treatment failure include low initial VDRL titers (<32 dilutions) and living in an area with a high incidence of infection with yaws [32].
For more than sixty years, the WHO has sought to exterminate one of the more treatable neglected tropical diseases. By using a single dose of oral azithromycin, ensuring treatment coverage with necessary follow-up surveillance, the rejuvenated interest in eradicating yaws is well underway with promising results. Reaching this goal by 2020 entails adequate funding and persistence. Rapid recognition and treatment of yaws can prevent irreversible gross deformities in young children. It is vital that clinicians maintain a high index of suspicion in any endemic area when presented with ambiguous skin lesions that mimics syphilis. With due diligence, yaws can be the only disease to be eradicated since small pox.
- Koff AB, Rosen T (1993) Nonvenereal treponematoses: yaws, endemic syphilis, and pinta. J Am Acad Dermatol 29: 519-535. [Crossref]
- Perine PL, Hopkins DR, Niemel PL (1984) Handbook of endemic treponematoses: yaws, endemic syphilis and pinta. Geneva: WHO
- Chin J (2000) Control of Communicable Diseases Manual. (17thedtn). American Public Health Association, Washington, DC, USA.
- WHO (2012) Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation. World Health Organization, Geneva, Switzerland.
- Mabey D (2012) Oral azithromycin for treatment of yaws. Lancet 379: 295-297. [Crossref]
- Rinaldi A (2012) Yaws eradication: facing old problems, raising new hopes. PLoS Negl Trop Dis 6: e1837. [Crossref]
- Thorpe VG (1898) YAWS in the SOUTH SEA ISLANDS. Br Med J 1: 1586. [Crossref]
- Fegan D, Glennon M, Macbride-Stewart G, Moore T (1990) Yaws in the Solomon Islands. J Trop Med Hyg 93: 52-57. [Crossref]
- Fegan D, Glennon MJ, Thami Y, Pakoa G (2010) Resurgence of yaws in Tanna, Vanuatu: time for a new approach? Trop Doct 40: 68-69. [Crossref]
- Anselmi M, Araujo E2021 Copyright OAT. All rights reservan RH (1995) Yaws in Ecuador: impact of control measures on the disease in the Province of Esmeraldas. Genitourin Med 71: 343-346. [Crossref]
- Mitjà O, Hays R, Lelngei F, Laban N, Ipai A, et al. (2011) Challenges in recognition and diagnosis of yaws in children in Papua New Guinea. Am J Trop Med Hyg 85: 113-116. [Crossref]
- Rinaldi A (2008) Yaws: a second (and maybe last?) chance for eradication. PLoS Negl Trop Dis 2: e275. [Crossref]
- Bora D, Dhariwal AC, Lal S (2005) Yaws and its eradication in India--a brief review. J Commun Dis 37: 1-11. [Crossref]
- WHO (2008) Elimination of Yaws in India. Weekly epidemiological record. 15:125-132.
- WHO (2013) Eradicating yaws: criteria and procedures for the verification of interruption of transmission and for certification of countries.
- [No authors listed] (2012) Eradication of yaws--the Morges strategy. Wkly Epidemiol Rec 87: 189-194. [Crossref]
- Asiedu K, Fitzpatrick C, Jannin J (2014) Eradication of yaws: historical efforts and achieving WHO's 2020 target. PLoS Negl Trop Dis 8: e3016. [Crossref]
- PubMed (2012) Yaws: Frambesia tropica. A.D.A.M. Medical Encyclopedia. PubMed Health, Atlanta, GA.
- Antal GM, Lukehart SA, Meheus AZ (2002) The endemic treponematoses. Microbes Infect 4: 83-94. [Crossref]
- Wicher K, Wicher V, Abbruscato F, Baughn RE (2000) Treponema pallidum subsp. pertenue displays pathogenic properties different from those of T. pallidum subsp. pallidum. Infect Immun 68: 3219-3225.
- [No authors listed] (2012) Yaws, a non-venereal treponemal infection. Still endemic in some parts of the world. Prescrire Int 21: 217-219. [Crossref]
- Castro LG (1994) Nonvenereal treponematosis. J Am Acad Dermatol 31: 1075-1076. [Crossref]
- Mitjà O, Hays R, Ipai A, Wau B, Bassat Q (2011) Osteoperiostitis in early yaws: case series and literature review. Clin Infect Dis 52: 771-774. [Crossref]
- Baker-Zander SA, Lukehart SA (1983) Molecular basis of immunological cross-reactivity between Treponema pallidum and Treponema pertenue. Infect Immun 42: 634-638. [Crossref]
- Engelkens HJ, Vuzevski VD, Stolz E (1999) Nonvenereal treponematoses in tropical countries. Clin Dermatol 17: 143-152. [Crossref]
- Marks M, Goncalves A, Vahi V, Sokana O, Puiahi E, et al. (2014) Evaluation of a rapid diagnostic test for yaws infection in a community surveillance setting. PLoS Negl Trop Dis 8: e3156. [Crossref]
- Ayove T, Houniei W, Wangnapi R, Bieb SV, Kazadi W, et al. (2014) Sensitivity and specificity of a rapid point-of-care test for active yaws: a comparative study. Lancet Glob Health 2: e415-421. [Crossref]
- Mitjà O, Hays R, Ipai A, Penias M, Paru R, et al. (2012) Single-dose azithromycin versus benzathine benzylpenicillin for treatment of yaws in children in Papua New Guinea: an open-label, non-inferiority, randomised trial. Lancet 379: 342-347. [Crossref]
- Thomson PDR (2002) Physician's Desk Reference. (56th edtn). Montvale, NJ, USA.
- Scolnik D, Aronson L, Lovinsky R, Toledano K, Glazier R, et al. (2003) Efficacy of a targeted, oral penicillin-based yaws control program among children living in rural South America. Clin Infect Dis 36: 1232-1238. [Crossref]
- Sehgal VN, Jain S, Bhattacharya SN, Thappa DM (1994) Yaws control/eradication. Int J Dermatol 33: 16-20. [Crossref]
- Mitjà O, Hays R, Ipai A, Gubaila D, Lelngei F, et al. (2011) Outcome predictors in treatment of yaws. Emerg Infect Dis 17: 1083-1085. [Crossref]











