Nanomedicine is the application of nanotechnology (the engineering of tiny machines) to the prevention and treatment of disease in the human body. Nanomaterials can impart antibacterial and anti-odour functionality on human skin in powder, gel, stick or spray underarm products. It has also antimicrobial and anti-irritant properties. This discipline is in its infancy. It has the potential to change medical science dramatically in the 21st century.
Molecular biomimetics: Nanotechnology through biology
Molecular biomimetics. This is the marriage of materials science engineering and molecular biology for development of functional hybrid systems, composed of inorganics and inorganic-binding proteins. The new approach takes advantage of DNA-based design, recognition,and self-assembly characteristics of biomolecules.
Traditional materials science engineering produces materials (for example, mediumcarbon steels depicted in the bright- and dark-field TEM images), that have been successfully used over the last century. Molecular biology focuses on structure– function relations in biomacromolecules, for example, proteins.
In molecular biomimetics, a marriage of the physical and biological fields, hybrid materials could potentially be assembled from the molecular level using the recognition properties of proteins under the premise that inorganic surface-specific polypeptides could be used as binding agents to control the organization and specific functions of materials.Molecular biomimetics simultaneously offers three solutions to the development of heterofunctional nanostructures.
(1) The first is that protein templates are designed at the molecular level through
genetics. This ensures complete control over the molecular structure of the protein template (that is, DNA-based technology).
(2) The second is that surface-specific proteins can be used as linkers to bind synthetic entities, including nanoparticles, functional polymers, or other nanostructures onto molecular templates (molecular and nanoscale recognition).
(3) The third solution harnesses the ability of biological molecules to self- and coassemble into ordered nanostructures. This ensures a robust assembly process for achieving complex nano-, and possibly hierarchical structures, similar to those found in nature (self-assembly).
The current knowledge of protein-folding predictions and surface-binding chemistries does not provide sufficiently detailed information to perform rational design of proteins. To circumvent this problem, massive libraries of randomly generated peptides can be screened for binding activity to inorganic surfaces using phage and cell-surface display techniques. It may ultimately be possible to construct a‘molecular erector’ set, in which different types of proteins, each designed to bind to a specific inorganic surface, could assemble into intricate, hybrid structures composed of inorganics and proteins. This would be a significant leap towards realizing molecularly designed, genetically engineered technological materials.
Selection of inorganic-binding proteins through display technologies
There are several possible ways of obtaining polypeptide sequences with specific affinity to inorganics. A number of proteins may fortuitously bind to inorganics, although they are rarely tested for this purpose. Inorganic-binding peptides may be designed using a theoretical molecular approach similar to that used for pharmaceutical drugs. This is currently impractical because it is time consuming and expensive. Another possibility would be to extract biomineralizing proteins from hard tissues followed by their isolation, purification and cloning. Several such proteins have been used as nucleators, growth modifiers, or enzymes in the synthesis of certain inorganics. One of the major limitations of this approach is that a given hard tissue usually contains many proteins, not just one, all differently active in biomineralization and each distributed spatially and temporally in complex ways.Furthermore, tissue-extracted proteins may only be used for the regeneration of the inorganics that they are originally associated with, and would be of limited practical use. The preferred route, therefore, is to use combinatorial biology techniques. Here, a large random library of peptides with the same number of amino acids, but of different sequences, is used to mine specific sequences that strongly bind to a chosen inorganic surface.
Since their inception, well-established in vivo combinatorial biology protocols (for example, phage display (PD) and cell-surface display (CSD)) have been used to identify biological ligands and to map the epitope (molecular recognition site) of antibodies. Libraries have also been screened for various biological activities, such as catalytic properties or altered affinity and specificity to target molecules in many applications including the design of new drugs, enzymes, antibodies, DNA-binding proteins and diagnostic agents. The power of display technologies relies on the fact that an a priori knowledge of the desired amino acid sequence is not necessary, as it can simply be selected and enriched if a large enough population of random sequences is available. In vitro methods, such as ribosomal and messenger RNA display technologies, have been developed for increased library size (1015) compared to those of in vivo systems (107–10).
Combinatorial biology protocols can be followed in molecular biomimetics to select polypeptide sequences that preferentially bind to the surfaces of inorganic compounds chosen for their unique physical properties in nano- and biotechnology. Libraries are generated by inserting randomized oligonucleotides within certain genes encoded on phage genomes or on bacterial plasmids (step 1 in Figure 1).
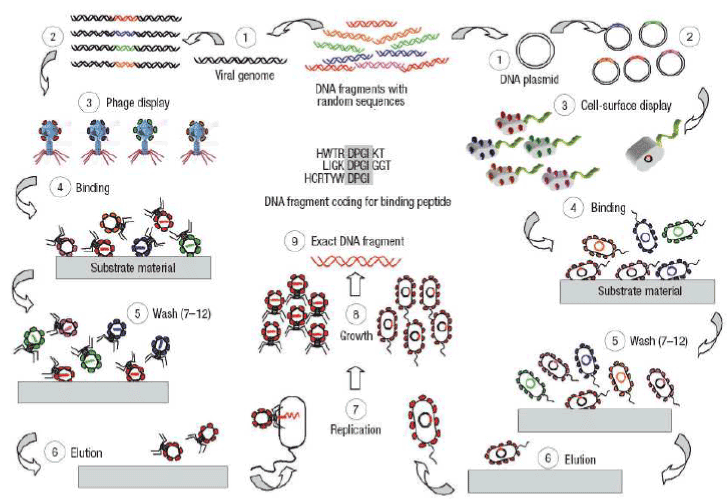
Figure 1. Phage display and cell-surface display. Principles of the protocols used for selecting polypeptide sequences that have binding affinity to given inorganic substrates. [Sarikaya, C. Tamerler, A.K.Y. Jen, K. Schulten F. Baneyx, Molecular biomimetics: nanotechnology through biology, Nature Materials, vol 2, 2003, p.p. 577-585].
This leads to the incorporation of a random polypeptide sequence within a protein residing on the surface of the organism (for example, the coat protein of a phage or an outer membrane or flagellar protein of a cell; step 2). The eventual result is that each phage or cell produces and displays a different, but random peptide (step 3). At this stage, a heterogeneous mixture of recombinant cells or phages are contactedwith the inorganic substrate (step 4). Several washing cycles of the phages or the cells eliminate non-binders by disrupting weak interactions with the substrate (step 5). Bound phages or cells are next eluted from the surfaces (step 6). In PD, the eluted phages are amplified by reinfecting the host (step 7). Similarly, in CSD, cells are allowed to grow (steps 7, 8). This step completes a round of biopanning. Generally, three to five cycles of biopanning are repeated to enrich for tight binders. Finally, individual clones are sequenced (step 9) to obtain the amino acid sequence of the polypeptides binding to the target substrate material.
- A Bio-Nanowire Device Interface (Figure 2)
Because the sizes of biological macromolecules are comparable to nanowire building blocks, these structures represent natural transducers for ultra-sensitive detection.
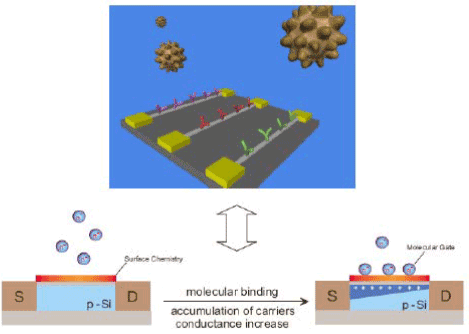
Figure 2. A Bio-Nanowire Device Interface
- Nanowire Nanosensors: Beginning (Figure 3): Cui, Wei, Park & Lieber: Science 293, 1289 (2001)
- Multiplexed Cancer Marker Detection (Figure 4): Zheng, Patolsky & Lieber, Nat. Biotech. 23, 1294 (2005).
- Undiluted Blood Serum Analysis (Figure 5): Serum samples are characterized after single step ‘desalting’purification. (1) Buffer; (2) Donkey Serum (DS), 59 mg/ml total protein; (3) DS + 2.5 pM PSA; (4) DS + 25 pM PSA (1) DS + 0.9 pg/ml; (2) DS. Marker proteins are detected selectively in presence of ca. 100-billion-fold excess of serum proteins.
- Nanoelectronic-Cell Interfaces (Figure 6):
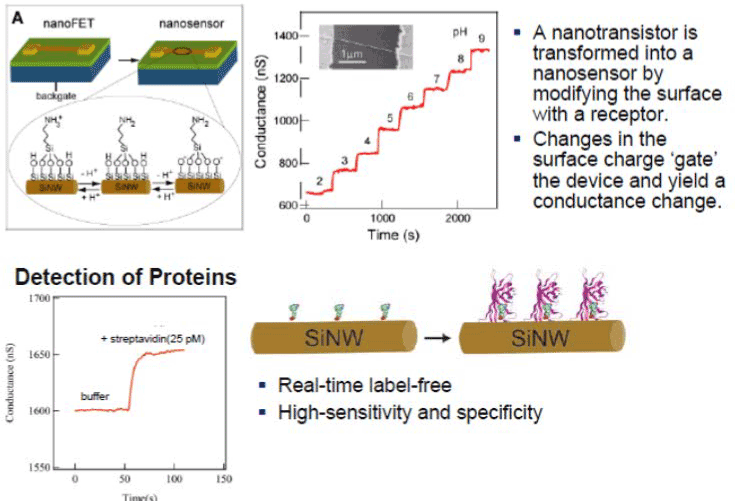
Figure 3. Nanowire Nanosensors: Beginning
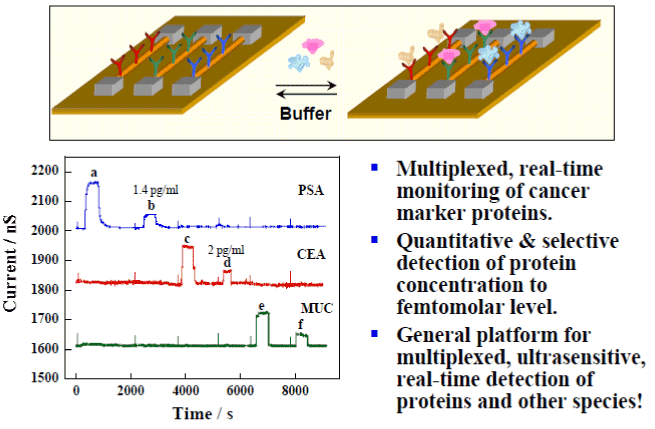
Figure 4. Multiplexed Cancer Marker Detection.
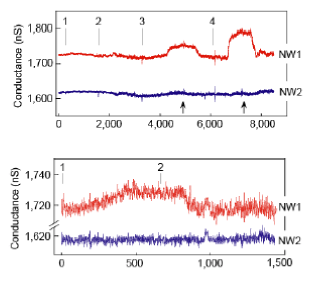
Figure 5. Marker proteins are detected selectively in presence of ca. 100-billion-fold excess of serum proteins.
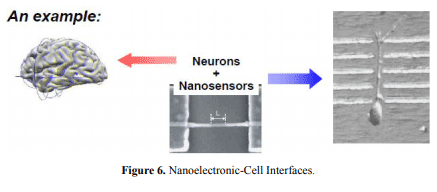
Figure 6. Nanoelectronic-Cell Interfaces.
Nanowire nanoelectronic devices can enable:
Interface to cells at natural scale of biological communication
Input/output of electrical signals
Input/output of chemical/biological signals
Conclusions
Nanoelectronic-Biological Interfaces Enable:
- Diagnostic devices for disease detection
- General detection & kinetics platform
- New tool for single-molecule detection/biophysic
- Powerful devices for electronic and chem/bio recording from cells, tissue & organs
- Potential implants for highly functional & powerful prosthetics, as well as hybrid biomaterials enabling new opportunities
2021 Copyright OAT. All rights reserv
Evaluating Research Motivation: Progress?
- Synthetic challenge of controlling structure and composition on many length scales
- Fundamental scientific questions in 1-dimensional systems
- Central importance of nanoscale wires in integrated nanosystems
- New/novel materials can make revolutionary vs. evolutionary changes in science and technology!
⇒ Many fundamental scientific questions remain, and will require bold researchers to address.
⇒ Pushing ourselves to identify and tackle these ‘big’ challenges, while difficult, offers the best opportunity to make revolutionary advances and benefit society!






