Background: Vascular endothelial growth factor (VEGF) plays an important role in the regulation of bone mineral metabolism by stimulation of osteoblast differentiation and survival.
Aims: We aimed to investigate the possible relation between bone mineral density (BMD) and VEGF gene 936 C>T and 2578 C>A single nucleotide polymorphisms (SNPs) in postmenopausal Turkish women.
Study design: Prospective, cross sectional, case control study
Methods: This study included 333 postmenopausal Turkish women, of whom 137 were osteoporotic (lumbar spine T score < -2.5 SD) and 196 were nonosteoporotic (lumbar spine T score > -1.5 SD). BMD measures were obtained using dual-energy X-ray absorptiometry. SNPs of the VEGF gene was examined by polymerase chain reaction-restriction fragment length method.
Results: TT genotype frequency of 936 C>T SNP of osteoporotic women was higher and AA genotype frequency of 2578 C>A SNP of osteoporotic women was lower than those of nonosteoporotic women. There was no significant difference between osteoporotic and nonosteoporotic women for frequencies of genotypes and alleles of two SNPs. For C(+936)T SNP; the mean height (p=0.007) and BMD (p=0.02) of TT genotype were significantly lower than those of CC and CT genotypes. For C(-2578)A SNP; the mean weight and BMD of AA genotype were higher than those of CC and CA genotypes. VEGF 936 CT genotype (OR= 7.58, 95% CI= 2.317 – 24.794, p<0.01) showed influence on lumbar spine BMD.
Conclusion: Phenotypic influence of heterozygous state of VEGF C936T polymorphism on our postmenopausal women is interesting. Extended population studies are needed to discuss our results.
postmenopausal osteoporosis, VEGF, bone mineral density, polymorphism
Postmenopausal osteoporosis, multifactorial and polygenic bone disease, is characterized by decreased bone mineral density (BMD) and increased fracture risk [1]. BMD has strong genetic determination with a high heritability of 50–80%. Population-based studies have identified polymorphisms in several candidate genes that have been associated with BMD [2,3]. The reduced BMD arises from the impaired balance between bone formation by osteoblasts and resorption by osteoclasts [1,4]. The vasculature and angiogenic factors play an important role in the regulation of BMD [4-8]. Vascular endothelial growth factor (VEGF) is one of the angiogenic factors that induces endochondral ossification and fracture healing [7,8]. VEGF has also been shown to play a role in the regulation of bone mineral metabolism by stimulation of osteoblast differentiation and survival [9]. In an animal model, it was suggested that impaired cartilage mineralization due to estrogen deficiency was caused by reduced expression of VEGF [10]. In an in vitro study it was observed that VEGF expression was increased with estradiol treatment in osteoblasts [11]. Griffith et al. observed reduced bone perfusion and decreased BMD in rats two weeks after ovariectomy [12]. Several hormones which influence skeletal homeostasis have been shown to regulate VEGF production locally [13,14]. And in this study we aimed to investigate the possible relation between BMD and VEGF gene 936 C>T and 2578 C>A single nucleotide polymorphisms (SNP) in postmenopausal Turkish women.
Subjects
This prospective, cross-sectional, case control study was conducted with 333 postmenopausal Turkish women who attended the Department of Nuclear Medicine of our hospital, between May 2009 and November 2009, after local ethical committee approval. The mean age, postmenopausal period, weight, height and body mass index of the study population were 57 ± 7 years, 9 ± 6 years, 70 ± 9 kg, 154 ± 5 cm and 29 ± 5 kg/m2 respectively. We organized this study with two groups. The first group (n = 137) was osteoporotic women whose lumbar spine T score was lower than -2.5 SD and the second group (n = 196) was non-osteoporotic women whose lumbar spine T score was greater than -1.0 SD. Careful physical examination and medical history review were done for all participants. Baseline blood analysis for liver- kidney function and fasting glucose were performed. The following criterias were used for exclusion: previous ovary surgery, diabetes mellitus, thyroid dysfunction, liver disease, any medication that affect bone metabolism. There was no difference among participants for ethnicity. Signed informed consents were taken during enrollment.
BMD measurement
Area BMD (g/cm2) at the lumbar spine L2–L4 and total hip was measured by dual energy X-ray absorptiometry (DEXA). Densitometers were calibrated daily. The coefficient of variation for the BMD was 0.54%.
SNP genotyping
Genomic DNA was extracted from peripheral venous blood by using the Wizard Genomic DNA Purification Kit (Promega, UK), according to the manufacturer’s instructions. VEGF C(-2578)A and C(+936)T single nucleotide polymorphisms (SNPs) were assessed. We used the protocol described by previous researchers [15,16]. Primer sequences and restriction products are shown in Figure-1. PCR cycling conditions were as follows: 94°C for 5 minutes, 35 cycles for 30 seconds at 94°C, 61°C for C(-2578)A, 62°C for C(+936)T, a final step at 72°C for 10 minutes to allow for the complete extension of all PCR fragment. PCR products were digested by Bgl II for C(-2578)A (rs6999947) polymorphism, and Hsp92II for C(+936)T (rs3025039) polymorphism at 37°C overnight, respectively. After digestion, digested products were seperated on a 3% agarose gel which were visualised by ethidium bromide.
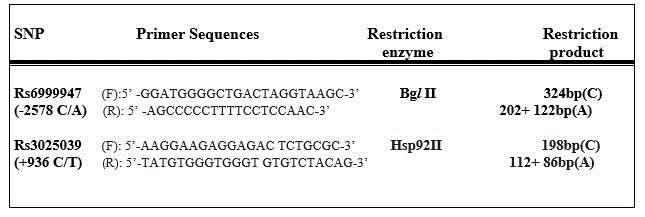
Figure 1. Primer Sequences and Protocols.
Statistical analysis
Statistical analysis was performed by using the Statistical Package for Social Science (SPSS) 16.0 (Inc., Chicago, IL, USA) version. Results were presented as mean and standard deviation or number and percentage, as appropriate. Differences between the means were analyzed by Student’s t-test and Mann–Whitney U-test according to the distribution of data. The significance of differences between the two groups was assessed using X2 test or Fisher’s exact test for categorical variables, where applicable. For detecting lumbar spine and total hip BMD of each SNP genotype, analysis of variance (ANOVA) was performed. Hardy–Weinberg equilibrium was tested for each genotyped SNP using chi-square statistics. Multinomial logistic regression was employed to determine the variables which have influence on BMD. In all examinations, a p value<0.05 was considered as statistically significant. Power analysis of the study was performed with program of G Power 3 and the power of our study was 81%.
The demographic characteristics of the study population were presented in Table 1. Lumbar spine BMD of osteoporotic and nonosteoporotic women were 0.801 ± 0.069 and 1.143 ± 0.263 g/cm2 respectively.
The C(+936)T and C(-2578)A genotype frequencies of whole population were respectively as follows: CC: 62.8%, CT: 30.3%, TT: 6.9%; CC: 32.7%, CA: 43.5%, AA: 23.7%. The C(+936)T and C(-2578)A genotype and allele frequencies of two groups were presented in Table 2. There was no significant difference between groups for frequencies of genotypes and alleles of two SNPs. But the frequency of homozygous mutant TT genotype of osteoporotic women was higher than of nonosteoporotic women; and frequency of homozygous mutant AA genotype of osteoporotic women was lower than of nonosteoporotic women. The frequencies of alleles were similar with Hardy– Weinberg equilibrium (X2=2.2; p=0.4). Variance analyses, performed for each genotype to determine the difference for the mean height, weight, BMI, lumbar spine BMD were presented in Table 3. For C(+936)T SNP; the mean height (p=0.007) and BMD (p=0.02) of TT genotype were significantly lower than those of CC and CT genotypes. For C(-2578)A SNP; the mean weight and BMD of AA genotype were higher than those of CC and CA genotypes.
Table 1. Demographic characteristics of all women in the study.
Parameters |
T score <-2.5
(n=137) |
T score > -1.0
(n=196) |
P value |
Age (years) |
58 ± 7 |
57 ± 7 |
0.15 |
Menopausal period (years) |
9 ± 7 |
8 ± 6 |
0.06 |
Weight (kg) |
70 ± 10 |
71 ± 9 |
0.08 |
Height (cm) |
153 ± 6 |
155 ± 5 |
0.09 |
BMI (kg/m2) |
29 ± 5 |
30 ± 5 |
0.23 |
Lomber BMD (g/cm2) |
0.801 ± 0.069 |
1.143 ± 0.263 |
<0.01 |
Smoking (%) |
25 |
27 |
0.54 |
Alcohol consumption (%) |
0.1 |
0.1 |
0.74 |
Daily calcium intake (mg) |
1100 ± 200 |
1150 ± 300 |
0.67 |
Note: Values are presented as mean ± SD and percent. BMI= body mass index; BMD= bone mineral density
Table 2. VEGF 936 C>T and 2578 C>A SNP genotype and allele frequencies.
|
T score <-2.5
n(%) |
T score >-1.5
n(%) |
P value |
OR |
95% CI |
C936T genotypes |
|
|
|
|
|
CC |
94 (68.6) |
115 (58.6) |
0.06 |
0.64 |
0.41-1.028 |
CT |
27 (19.7) |
74 (37.8) |
|
|
|
TT |
16 (11.7) |
7 (3.6) |
|
|
|
Total |
137 (100) |
196 (100) |
|
|
|
C936 T alleles |
|
|
|
|
|
C |
215 (78.5) |
304 (77.5) |
0.26 |
0.85 |
0.64-1.025 |
T |
59 (21.5) |
88 (22.5) |
|
|
|
Total |
274 (100) |
392 (100) |
|
|
|
C2578A genotypes |
|
|
|
|
|
CC |
46 (33.6) |
63 (32.1) |
0.78 |
0.93 |
0.589-1.491 |
CA |
67 (48.9) |
78 (39.8) |
|
|
|
AA |
24 (17.5) |
55 (28.1) |
|
|
|
Total |
137 (100) |
196 (100) |
|
|
|
C2578A alleles |
|
|
|
|
|
C |
159 (58) |
204 (52) |
0.38 |
0.54 |
0.45-1.120 |
A |
115 (42) |
188 (48) |
|
|
|
Total |
274 |
392 |
|
|
|
Table 3. Variance analysis of genotypes.
|
936CC |
936CT |
936TT |
P value |
2578CC |
2578CA |
2578AA |
P value |
Weight (kg) |
71 ± 13 |
72 ± 12 |
67 ± 16 |
0.33 |
71 ± 11 |
71 ± 14 |
73 ± 13 |
0.66 |
Height (cm) |
155 ± 61 |
155 ± 61 |
150 ± 82 |
0.007 |
154 ± 6 |
155 ± 6 |
156 ± 6 |
0.26 |
BMI (kg/m2) |
29 ± 5 |
30 ± 5 |
29 ± 6 |
0.81 |
30 ± 4 |
29 ± 5 |
30 ± 5 |
0.96 |
Lomber BMD (g/cm2) |
1.005 ±
0.2931 |
1.022 ±
0.2141 |
0.885 ±
0.1762 |
0.02 |
1.002 ±
0.247 |
0.992 ±
0.243 |
1.021 ±
0.328 |
0.21 |
Note: Values are presented as mean ± SD. BMI= body mass index; BMD= bone mineral density. The differences between values are shown as superscript numbers.
Multinomial logistic regression analysis (Table 4) showed that menopausal period (OR= 0.87, 95% CI= 0.805 – 0.944, p<0.01) and VEGF 936 CT genotype (OR= 7.58, 95% CI= 2.317 – 24.794, p<0.01) have had influence on lumbar spine BMD.
Table 4. Multinomial logistic regression analysis of factors that have influence on lumbar spine bone mineral density.
Parameter |
OR |
95% Confidence Interval |
P value |
Age |
1.02 |
0.963 – 1.100 |
0.42 |
Weight |
1.01 |
0.714 – 1.419 |
0.96 |
Height |
1.05 |
0.766 – 1.450 |
0.74 |
Menopausal period |
0.87 |
0.805 – 0.944 |
<0.01 |
Alcohol consumption |
0.98 |
0.93 – 1.008 |
0.24 |
Daily calcium intake |
0.95 |
0.88 – 1.020 |
0.31 |
VEGF 936 CC genotype |
2.53 |
0.845 – 7.611 |
0.09 |
VEGF 936 CT genotype |
7.58 |
2.317 – 24.794 |
<0.01 |
VEGF 2578 CC genotype |
0.57 |
0.281 – 1.193 |
0.13 |
VEGF 2578 CA genotype |
0.56 |
0.284 – 1.130 |
0.11 |
In the present study we tested whether VEGF C936T and C2578A SNPs influence lumbar spine BMD in postmenopausal osteoporotic and nonosteoporotic Turkish women. While the frequency of VEGF 936 TT mutant genotype was high, frequency of 2578 AA mutant genotype was low in osteoporotic women. VEGF 936 CT genotype showed significant influence on lumbar spine BMD. Phenotypic influence of a heterozygous state of VEGF C936T polymorphism is interesting. Costa et al. [17] investigated VEGF C936T SNP on 252 postmenopausal Caucasian women (136 osteoporotic and 116 nonosteoporotic). The genotype distribution of their population were as follows; CC: 75.8%, CT: 21.5% and TT: 2.7%. They reported no significant difference in allele frequencies between osteoporotic and nonosteoporotic women. The frequency of C936T genotype of our whole population showed concordance with the results of Costa et al. [17].
Bone vasculature plays an important role in bone remodeling. Bone remodeling involve the interaction between angiogenic and osteogenic pathways. This intimate relation between bone formation and vascularization has been termed as angiogenic–osteogenic coupling [18]. The formation of new blood vessels in the metabolically active bone tissue is required for supplying nutrients, oxygen, growth factors, cytokines and osteoblast and osteoclast precursors [19)] VEGF is an endothelial cell survival factor and is required for effective coupling of angiogenesis and osteogenesis [20,21]. Horner et al. [22] investigated the weak expression of VEGF in the hypoxic region chondrocytes of human neonatal growth plates which promoted the invasion of the cartilage by metaphyseal vessels resulting in new bone formation. This was pointed out in a study where blocked VEGF receptors resulted in the suppression of vascular invasion of the cartilage and of bone formation (6). Investigators observed the increased protein expression of VEGF in osteoblasts by dose- and time-dependent estradiol treatment at in vitro conditions [23,24]. Inadequate blood flow has been linked to osteoporosis [19]. Mice with VEGF-deficient osteoblastic lineage cells demonstrate age-dependent loss of bone mass [25].
Ding et al. [26] investigated the relation between blood supply and ovariectomy induced osteoporosis in a mice model. Sixty mice were randomly divided into an ovariectomy group (n=30) and a control group (n=30). Four weeks after ovariectomy, immunohistochemically studied VEGF expression on tibial metaphysis was significantly decreased. Neve et al. [27] studied in vitro differences in VEGF production and expression of cultured human osteoblastic cells derived from healthy donors and from osteoporotic subjects. They observed that normal and pathological osteoblasts produced and expressed VEGF and pathological osteoblasts produced a strong angiogenic response greater than normal cells. Investigators indicated that mice with conditional VEGF deficiency in osteoblastic precursor cells manifested an osteoporosis-like phenotype characterized by reduced bone mass and increased bone marrow fat [28]. A significant decrease in blood vessel volume and expression of VEGF protein at the distal femur was observed in ovariectomized mice [29]. Similarly, in bone repair, numerous studies have shown that impairments in VEGF signalling are associated with deficiencies in new bone formation [30-32].
A lot of stimulators can modulate VEGF production by bone tissue including hormonal, mechanical and environmental influences. Serum levels of VEGF were demonstrated to be higher in ovariectomy performed mice compared to nonovariectomy performed mice. In ovariectomy performed mice, trabecular bone volume of the femur was reduced, and amount of osteoclasts was significantly rised. VEGF antagonist treatment after ovariectomy blocked osteoclast increament in mice [33]. In the study of Mao-wei et al., anti-hyperlipemic medication (fluvastatine) was given in osteoporotic rats and it was stated that fluvastatine can be effective in osteoporotic fracture healing by increasing VEGF levels [34]. Researhers studied the effect of VEGF on the recovery of bone drilling defects in rat femur delivered with first-generation adenoviral vector. They injected virus into the muscle layer surrounding the bone drilling defect and they followed healing for 1, 2, and 4 weeks. VEGF over expression stimulated periosteal cartilage healing [35]. Another researchers suggest that adenovirus-mediated VEGF gene transfer induces bone formation by increasing osteoblast activity and may be useful for the treatment of osteoporosis and other diseases that require efficient osteogenic therapy [36].
In conclusion, in our population, TT mutant genotype of 936 C>T polymorphism and AA mutant genotype of 2578 C>A polymorphism did not show influence on BMD. But interestingly CT heterozygous genotype of VEGF 936 C>T polymorphism showed interaction with BMD. Of course our results must be discussed with expended population studies and expression studies.
This study was supported by Firat University Scientific Research Foundation (FÜBAP).
All authors declare that they have no conflict of interest to disclose.
- Ralston SH (2007) Genetics of osteoporosis. Proc Nutr Soc 66: 158-165. [Crossref]
- Deng HW, Chen WM, Conway T, Zhou Y, Davies KM, et al. (2000) Determination of bone mineral density of the hip and spine in human pedigrees by genetic and life-style factors. Genet Epidemiol 19: 160-177. [Crossref]
- Deng HW, Stegman MR, Davies KM, Conway T, Recker RR (1999) Genetic determination of variation and covariation of peak bone mass at the hip and spine. J Clin Densitom 2: 251-263. [Crossref]
- Parfitt AM (2000) The mechanism of coupling: a role for the vasculature. Bone 26: 319-323. [Crossref]
- Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F (2000) Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci 113: 59-69. [Crossref]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, et al. (1999) VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5: 623-628. [Crossref]
- Petersen W, Tsokos M, Pufe T (2002) Expression of VEGF121 and VEGF165 in hypertrophic chondrocytes of the human growth plate and epiphyseal cartilage. J Anat 201: 153-157. [Crossref]
- Pufe T, Wildemann B, Petersen W, Mentlein R, Raschke M, et al. (2002) Quantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: a study in the rat. Cell Tissue Res 309: 387-392. [Crossref]
- Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, et al. (2000) Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 141: 1667-1674. [Crossref]
- Pufe T, Claassen H, Scholz-Ahrens KE, Varoga D, Drescher W, et al. (2007) Influence of estradiol on vascular endothelial growth factor expression in bone: a study in Göttingen miniature pigs and human osteoblasts. Calcif Tissue Int 80: 184-191. [Crossref]
- Liu XD, Cai F, Liu L, Zhang Y, Yang AL (2015) MicroRNA-210 is involved in the regulation of postmenopausal osteoporosis through promotion of VEGF expression and osteoblast differentiation. Biol Chem 396: 339-347. [Crossref]
- Griffith JF, Wang YX, Zhou H, Kwong WH, Wong WT, et al. (2010) Reduced bone perfusion in osteoporosis: likely causes in an ovariectomy rat model. Radiology 254: 739-746. [Crossref]
- Esbrit P, Alvarez-Arroyo MV, De Miguel F, Martin O, Martinez ME, et al. (2000) C-terminal parathyroid hormone-related protein increases vascular endothelial growth factor in human osteoblastic cells. J Am Soc Nephrol 11: 1085-1092. [Crossref]
- Hyder SM, Nawaz Z, Chiappetta C, Stancel GM (2000) Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res 60: 3183-3190. [Crossref]
- Liu Q, Li Y, Zhao J, Sun DL, Duan YN, et al. (2009) Association of polymorphisms -1154G/A and -2578C/A in the vascular endothelial growth factor gene with decreased risk of endometriosis in Chinese women. Hum Reprod 24: 2660-2666. [Crossref]
- Garza-Veloz I, Castruita-De la Rosa C, Cortes-Flores R, Martinez-Gaytan V, Rivera-Muñoz JE, et al. (2011) No association between polymorphisms/haplotypes of the vascular endothelial growth factor gene andpreeclampsia. BMC Pregnancy Childbirth 11: 35.
- Costa N, Paramanathan S, Mac Donald D, Wierzbicki AS, Hampson G (2009) Factors regulating circulating vascular endothelial growth factor (VEGF): association with bone mineral density (BMD) in post-menopausal osteoporosis. Cytokine 46: 376-381. [Crossref]
- Maes C (2013) Role and regulation of vascularization processes in endochondral bones. Calcif Tissue Int 92: 307-323. [Crossref]
2021 Copyright OAT. All rights reserv
- Saran U, Gemini Piperni S2, Chatterjee S3 (2014) Role of angiogenesis in bone repair. Arch Biochem Biophys 561: 109-117. [Crossref]
- Clarkin CE, Gerstenfeld LC (2013) VEGF and bone cell signalling: an essential vessel for communication? Cell Biochem Funct 31: 1-11. [Crossref]
- Chim SM, Tickner J, Chow ST, Kuek V, Guo B, et al. (2013) Angiogenic factors in bone local environment. Cytokine Growth Factor Rev 24: 297-310. [Crossref]
- Horner A, Bishop NJ, Bord S, Beeton C, Kelsall AW, et al. (1999) Immunolocalisation of vascular endothelial growth factor (VEGF) in human neonatal growth plate cartilage. J Anat 194: 519-524. [Crossref]
- Liu XD, Cai F, Liu L, Zhang Y, Yang AL (2015) MicroRNA-210 is involved in the regulation of postmenopausal osteoporosis through promotion of VEGF expression and osteoblast differentiation. Biol Chem 396: 339-347. [Crossref]
- Liu X, Tu Y, Zhang L, Qi J, Ma T, et al. (2014) Prolyl hydroxylase inhibitors protect from the bone loss in ovariectomy rats by increasing bone vascularity. Cell Biochem Biophys 69: 141-149. [Crossref]
- Liu Y, Olsen BR (2014) Distinct VEGF functions during bone development and homeostasis. Arch Immunol Ther Exp (Warsz) 62: 363-368. [Crossref]
- Ding WG, Wei ZX, Liu JB (2011) Reduced local blood supply to the tibial metaphysis is associated with ovariectomy-induced osteoporosis in mice. Connect Tissue Res 52: 25-29. [Crossref]
- Neve A, Cantatore FP, Corrado A, Gaudio A, Ruggieri S, et al. (2013) In vitro and in vivo angiogenic activity of osteoarthritic and osteoporotic osteoblasts is modulated by VEGF and vitamin D3 treatment. Regul Pept 184: 81-84. [Crossref]
- Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, et al. (2012) Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest 122: 3101-3113. [Crossref]
- Zhao Q, Shen X, Zhang W, Zhu G, Qi J, et al. (2012) Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone 50: 763-770. [Crossref]
- Street J, Bao M, deGuzman L, Bunting S, Peale FV Jr, et al. (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 99: 9656-9661. [Crossref]
- Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, et al. (2005) Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res 20: 1114-1124. [Crossref]
- Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, et al. (2005) VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res 20: 2017-2027. [Crossref]
- Kodama I, Niida S, Sanada M, Yoshiko Y, Tsuda M, et al. (2004) Estrogen regulates the production of VEGF for osteoclast formation and activity in op/op mice. J Bone Miner Res 19: 200-206. [Crossref]
- Mao-wei Y, Yue Z, Guan-jun T, Gang L (2007) Effect of fluvastatin on vascular endothelial growth factor in rats with osteoporosis in process of fracture healing. Chin J Traumatol 10: 306-310. [Crossref]
- Tarkka T, Sipola A, Jämsä T, Soini Y, Ylä-Herttuala S, et al. (2003) Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med 5: 560-566. [Crossref]
- Hiltunen MO, Ruuskanen M, Huuskonen J, Mähönen AJ, Ahonen M, et al. (2003) Adenovirus-mediated VEGF-A gene transfer induces bone formation in vivo. FASEB J 17: 1147-1149. [Crossref]

