Interleukin-10 is the prototype of the anti-inflammatory cytokines. Its inhibitory action is exerted primarily towards the most typical markers of inflammation, such as IL-1; IL-6; TNF-α; GM-CSF and IFN-γ. The immunomodulatory action of IL-10 has meant that it was immediately regarded as a potential therapeutic tool for diseases in both acute and chronic inflammatory basis as well as in autoimmune diseases with inflammatory component. Recombinant human IL-10 has been tested in healthy volunteers, patients with Crohn's disease, rheumatoid arthritis, psoriasis, hepatitis C, HIV. The results obtained showed a positive immunomodulatory capacity of IL-10 but also underlined significant critical points, in particular the dose-dependent side effects.
These pitfalls can be avoided by s the use of low-dose IL-10 prepared according to the Sequential Kinetic Activation (SKA) method.
interleukin 10 (il-10), low dose medicine (ldm), sequential kinetic activation (ska), inflammatory diseases, autoimmune diseases, th1/th2 balance
Interleukin-10 (IL-10) is a homodimeric cytokine which modulates the biological activities of immune cells, keratinocytes and endothelial cells. IL-10 binds to a tetrameric transmembrane cytokine receptor composed of two molecules of IL-10R1 and two accessory molecules of IL-10R2. IL-10-receptor interaction starts an intracellular signaling pathway that involves JAK1, Tyk2 and STAT3; STAT3 dimerization and nuclear translocation induce the expression of target genes (Figure 1). No compensatory pathways exist, the IL-10-receptor interaction loss of function results in signaling failure [1,2].
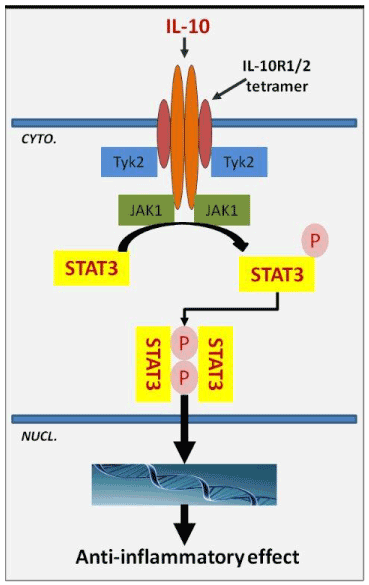
Figure 1. Schematic representation of IL-10 interaction mechanism with the specific receptor. IL-10 binding starts an intracellular signaling pathway involving STAT3 as key translocation nuclear factor which induces the activation of specific gene encoding for anti-inflammatory factors.
IL-10 is the prototype of anti-inflammatory cytokines, discovered in late ‘80s and originally classified as a key factor in the inhibition of cytokine synthesis; it is produced by various cell types including lymphocytes, monocytes and macrophages, and its inhibitory action is exerted mainly against the most typical markers of inflammation such as IL-1; IL-6; TNF-α; GM-CSF and IFN-γ [3,4].
IL-10 exerts its modulating effects preferentially on both resident and circulating immune cells; the major effect of IL-10 is the regulation of the Th1/Th2 balance; Th1 cells are involved in cytotoxic T-cell responses whereas Th2 cells regulate B-cell activity [5].
Interestingly, IL-10 is able to inhibit both the Th1-type and the Th2-type responses but the effect on Th1 subpopulation is predominant; IL-10 is also a promoter of Th2 response (pleiotropic effect) by inhibiting IFN-γ production from Th1 cells via IL-12 synthesis suppression in accessory cells (Figure 2).
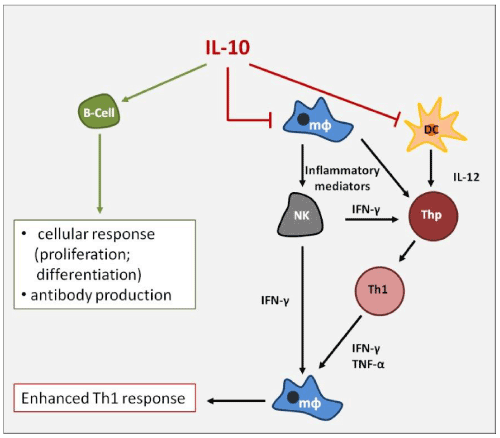
Figure 2. IL-10 is able to modulate the immune reactivity activating the cellular response via B-Cells and inhibiting the IFN-γ- mediated Th1 response.
IL-10 is not a merely suppressive agent. IL-10 not only inhibits the production of pro-inflammatory mediators but also augments the production of anti-inflammatory factors including soluble TNF-α receptors and IL-1RA. IL-10 down-regulates the expression of MHC class II molecules (both constitutive and IFN-γ-induced), as well as that of co-stimulatory molecule, CD86, and adhesion molecule, CD58. It is an inhibitor of IL-12 production from monocytes, which is required for the production of specific cellular defense response [6].
The first studies on the immunomodulatory effects of IL-10 were conducted using as a model the stimulation of immune cells of the peripheral blood with LPS which simulates ex vivo the state of endotoxemia typical of bacterial sepsis. This model has the advantage of representing an unspecific inflammatory stimulus, intense enough to trigger the inflammatory response supported by the three fundamental pro-inflammatory cytokines: IL-1 Β; TNF-α and IL-6 [7,8].
These cytokines are responsible for the onset and the maintenance of the inflammatory state in many diseases. Both IL-1 Β and TNF-α, induce the production of adhesion molecules, chemokines, growth factors and lipid mediators such as prostaglandins and nitric oxide (NO). These mediators stimulate leukocyte recruitment at the site of inflammation by amplifying the mechanisms of innate immunity. IL-6 is instead a secondary mediator, responsible for the maintenance of the inflammatory response and stimulates the production of acute phase proteins in the liver.
The physiological increase in the levels of IL-10 after about 72 hours from the starting of the inflammatory response leads to a progressive resolution of the inflammatory event [9].
Thinking about the intestinal immune homeostasis IL-10 is the key down-regulator of the immune system; IL-10 modulating activity prevents hyper-activation of immune response and maintains the so called physiological inflammation.
The role of IL-10 in maintaining homeostatic conditions is the cardinal point of its hypothetical therapeutic uses; the years 1995, 1996 and 1997 represent three milestones in the history of the study use of recombinant human interleukin 10 (rhIL-10) therapeutic tool. In 1995 the results of a phase 1 study conducted in healthy patients regarding the administration of rhIL-10 in a single solution iv at various doses are in published by Chernoff and colleagues; a single intravenous injection of IL-10 was considered safe in humans, exerted an inhibitory effects on T cells and suppressed the production of the pro-inflammatory cytokines TNF-a and IL-1 [10].
In 1996 Huhn and colleagues published an article in which they traced the first description of the pharmacokinetics and immunomodulatory properties of IL-10. The administration of a single dose of IL-10 intravenously (minimum dose 0.1 µg; maximum dose 100 µg) was performed on normal volunteers and IL-10 showed a dose-dependent ability to modulate IL-1 Β and TNF-α expression after LPS challenge on ex vivo whole blood samples. At higher doses the single IL-10 administration showed moderate flu-like side effects [11].
The third study, a randomized, double-blind, placebo-controlled, clinical trial conducted by Van Deventer and colleagues and published in 1997, demonstrated the efficacy of repeated doses of rhIL-10 i.v. for the treatment of Crohn's disease refractory to steroids [12].
These works represent the cornerstones for the study of the biological functions of IL-10 and its therapeutic applications; recombinant human IL-l0 showed potential clinical interest as an immune system modulator in diseases with an important inflammatory component such as autoimmune diseases, inflammatory bowel diseases, Based on its immunoregulatory function, IL-10 is a tempting candidate for therapeutic intervention in a wide variety of disease states, including autoimmune disorders, acute and chronic inflammatory diseases, cancer, infectious disease, autoimmune and allergic disease, rejection of transplanted organs and graft-versus-host diseases after transplantation.
The maintenance of the physiological balance between the various pro and anti-inflammatory cytokines is essential for the proper functioning of the mechanisms of the immune response. The large spectrum of action of IL-10 at the level of the immune system leads to consider this cytokine a very sensitive check-point of the immune response; deviations from homeostatic values of IL-10 are related to the onset of diseases with immunological component.
IL-10 down-regulation
The lack of IL-10 is connected to hypersensitivity reactions and typical of diseases such as rheumatoid arthritis, psoriasis and other autoimmune Th1-dependent diseases or inflammatory forms such as colitis and Crohn's disease [13].
In psoriasis for example low levels of IL-10 are detectable both in the skin by immunohistochemistry both through direct dosing into the fluid of the psoriatic lesion. In perfect agreement with the cytokine profile the prevalence of Th1 subpopulation of cells within the T cell set is assessed [14,15].
IL-10 plays an important role even in allergic contact dermatitis and in non-atopic eczema. The allergic contact dermatitis have a strong Th1-type immune response associated with low levels of IL-10 [16] while in the non-atopic eczema has been shown that the effective treatment with UV light induces the infiltration of macrophages with release of IL-10 [17].
In inflammatory bowel diseases the role of IL-10 is particularly important in managing the Th1/Th2 switch which is directly involved in the maintenance of physiologic inflammation between the microbiota- and food-derived antigens and antigens with pathogenic origin. This fundamental equilibrium is called immune tolerance and an alteration in the physiological expression of IL-10 induces an imbalance in this system with consequent appearance of inflammatory phenomena [18,19].
Th1/Th2 imbalance is also connected with vascular walls chronic inflammation secondary to dyslipidemia, diabetes and other metabolic and inflammatory diseases. Low levels of Th2 cytokines (including IL-10) are detected in atherosclerotic lesions [20] and, on the contrary, typical Th1/Th17 cytokines such as IL-6, IL-17 and TNF-α are over-expressed.
Reduced vascular endothelial function is directly linked with IL-10 down-regulation [21]; decreasing levels of IL-10 are deeply involved in age-related vascular loss-of-function and in increased cardiovascular diseases susceptibility [22].
IL-10 up-regulation
IL-10 up-regulation is linked with the increased chance of cancer development, viral infection chronicization and Th2 dependent autoimmune, inflammatory and allergic disorders onset [23-25].
IL-10 is over-expressed in malignant diseases such as melanoma, carcinoma and lymphoma. In solid tumors high levels of circulating IL-10 are linked with disease progression, metastasize and immune suppression, in particular T cell-mediated immune response is bypassed in cutaneous malignances; tumor cells derived from NK, T and B cell lymphomas produce an active form of IL-10. There is also a correlation between high levels of IL-10 and VEGF over-expression in some types of esophageal cancer, probably due to the angiogenic activity exerted by IL-10. Increased IL-10 levels are linked with poor prognosis, unresponsiveness to chemotherapy and tumor recurrence after surgery [23].
IL-10 up-regulation leads to an inappropriate clearance of pathogens, in particular viruses such as HIV, HBV, HCV, EBV and HPV perform immune escape strategies based on immune suppression, in fact IL-10 reduces NK cytotoxicity and Th-1 response resulting in intense immune depression. Some viruses (e.g. EBV) [24] are also able to encode for a viral form of IL-10 which contribute to infection chronicization creating an immunotolerant environment.
Th2-driven allergic response is strictly linked with IL-10 over-expression which induces a type I hypersensitivity characterized by an increased production of IL-4 and IL-5 cytokines and IgE antibodies. The role of IL-10 up-regulation as inducer of Th2 response is recognized as pivotal in food allergy, asthma, eosinophilic esophagitis and atopic dermatitis [13,25,26].
The relevant immunomodulating properties of IL-10 and its proved efficacy in experimental models of inflammatory diseases lead this cytokine to be an important tool for an hypothetical therapeutic use. Preliminary tests on healthy volunteers revealed an acceptable picture of safety presenting dose-dependent side effects culminating in flu-like episodes and transient neutrophilia, lymphocytopenia, monocytosis, and a delayed reversible decrease in platelet counts [11,26,27].
The relevant immunomodulating properties of IL-10 and its proved efficacy in experimental models of inflammatory diseases lead this cytokine to be an important tool for an hypothetical therapeutic use. Preliminary tests on healthy volunteers revealed an acceptable picture of safety presenting dose-dependent side effects culminating in flu-like episodes and transient neutrophilia, lymphocytopenia, monocytosis, and a delayed reversible decrease in platelet counts [11,26,27].
One of the first therapeutic applications of IL-10 has been experimented in the treatment of patients suffering from Crohn's disease resistant to steroids. The results of the various trials have shown some significant problems as the extreme variability of response to treatment and the need for relevant doses of recombinant protein. The latter aspect in particular has resulted in the emergence of a serious side effect such as anemia due to iron sequestration induced by ferritin and soluble receptor for transferrin over-expression. Overall, no study on the use of high doses of IL-10 for the treatment of Crohn's disease has produced results that would suggest its approval for use in therapy [12,31-34].
Many studies focused on the use of recombinant human IL-10 in psoriasis; the imbalance of the immune response towards a pro-inflammatory Th1-type-dependent characteristic of psoriasis is the therapeutic target that justifies the experimental use of rhIL-10. Since 1998, the results of several clinical trials have been published. The results of the use of IL-10 via subcutaneous injection are variable, on one side there is an effective activity in improving the picture of the disease (typically an improvement in the disease activity index PASI -Psoriasis Area Severity Index-) on the other side there was a marked variability in the response of the individual patient and a short duration of effect [35-37].
The side effects that occur in the treatment of psoriasis with rhIL-10 are essentially the same as described in the treatment of Crohn's disease; anemia, thrombocytopenia and reduction of serum cholesterol were recorded in the medium term treatment with rhIL-10.
The issues related to the use of rhIL-10 are also reflected on the hypothetical use of high doses of other cytokines and signaling molecules such as hormones, antibodies and neurotransmitters. In particular the dose-dependence of the side effects and the consequent difficulty of regulating in a refined manner the immunomodulatory response represent the most relevant pitfalls, even in the face of effectiveness evidence.
Recently it has been proposed a new approach for the use of signaling molecules for therapeutic purposes. It is based on the use of low doses of cytokines, antibodies, neurotransmitters and hormones set up using an innovative pharmaceutical technology called Sequential Kinetic Activation (SKA); a drug delivery system which allows the nano-concentrations to be active even below the actually considered minimum effective dose with therapeutic results comparable to those induced by high concentrations; this strategy is the core of Low Dose Medicine (LDM), an innovative medical paradigm born from the fusion of the most recent knowledge in the fields of molecular biology, Psycho-Neuro-Endocrine-Immunology (PNEI) and nano-concentrations research.
From a clinical point of view, the hypothetical therapeutic approach is to use antagonistic low dose molecules in order to re-equilibrate a biological effect according to the principle of “opposing” molecules. Obviously, IL-10 represents a lead molecule for Th1/Th2 rebalance under LDM point of view.
Scientific research has validated the theoretical principles of LDM [38-41]. Referring to IL-10 in 2013 a paper was published describing how low doses of SKA IL-10 (in association with low doses of SKA anti-IL-1 antibody) were able to improve gut morphology and functionality in a murine model of IBDs [40]. Recently (2014) a clinical study regarding the use of low dose cytokines for the treatment of Psoriasis Vulgaris was published [42]. The authors investigated the possibility of using specific low dose cytokines (IL-4; IL-10; IL-11) for the psoriasis treatment. The efficacy of treatment with low-dose cytokines was evaluated both in terms of improvement of the condition of psoriatic lesions and in the quality of life through a multicenter double-blind placebo-controlled clinical study of a significant number of patients and conducted through the use of internationally validated rating scales PASI and DLQI (Dermatology Life Quality Index) for the evaluation of the extent of the lesions and to determine the quality of life respectively. The obtained results allowed to identify some key points on the activity of the tested cytokines on psoriasis vulgaris: they are effective and safe from a therapeutic point of view and also have a long-term action, which extends into the first months after the end of treatment. This feature may be crucial in view of the treatment of chronic diseases.
Several problems have substantially prevented IL-10 to move from testing phases to clinical practice; they are mainly associated with:
- • the need to use relevant doses of recombinant interleukin
- • the route of administration (usually systemic, intravenous or subcutaneous)
- • the costs of production of the cytokine itself.
The use of high doses of rhIL-10 for therapeutic purposes collides with the inevitable side effects highlighted by studies conducted over 20 years in spite of the unquestionable ability of regulating the immune system.
From a point of view of the seriousness, severe acute episodes of adverse effects are not recorded but it is also evident how the alteration of the haematic picture and the immunosuppression phenomena (although disappear with discontinuation of therapy) are incompatible with the use of IL-10 in the control of chronic diseases such as most of those in which the imbalance Th1/Th2, elective IL-10 applicative field, is present. Another critical factor is the production of auto-antibodies anti-IL-10, which restricts the efficacy of the treatment and further exclude the possibility of a long-term use which is desirable for the treatment of chronic inflammatory diseases and inflammation-related disorders such as cardiovascular ones.
Finally, from the point of view of patient compliance the injective administration route both iv and subcutaneous is certainly not appreciated.
These limits, strongly related to the high dosage, can be eluded thanks to LDM approach application and the use of low-dose IL-10 (and others interleukins or signal molecules) prepared according to the method SKA. The use of low dose SKA IL-10 and molecules (the operative concentration is in the order of femtogram/ml) overcomes the dose-dependent side effect while ensuring the effectiveness of the action and the ability to perform long-term therapy.
- Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B (2011) IL-10 and IL-10 receptor defects in humans. Ann N Y Acad Sc. 1246: 102-7. [Crossref]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, et al. (2004) Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 22: 929-79. [Crossref]
- Bijjiga E, Martino AT (2013) Interleukin 10 (IL-10) Regulatory Cytokine and its Clinical Consequences. J Clin Cell Immunol S1: 007.
- Striz I, Brabcova E, Kolesar L, Sekerkova A (2014) Cytokine networking of innate immunity cells: a potential target of therapy. Clin Sci (Lond) 126(9): 593-612. [Crossref]
- Beebe AM, Cua DJ, de Wa2021 Copyright OAT. All rights reservleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev 13: 403-12. [Crossref]
- Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH (1997) Induction of tolerance by IL-10-treated dendritic cells. J Immunol 159: 4772-80. [Crossref]
- Cöl R, Durgun Z (2011) Effect of recombinant interleukin-10 on some haematological and biochemical parameters in a rat endotoxaemic model. Acta Vet Hung 59: 237-45. [Crossref]
- Pajkrt D, Camoglio L, Tiel-van Buul MC, de Bruin K, Cutler DL, et al. (1997) Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: effect of timing of recombinant human IL-10 administration. J Immunol 158: 3971-7. [Crossref]
- Petersen AM, Pedersen BK (1985) The anti-inflammatory effect of exercise. J Appl Physiol 98: 1154-62. [Crossref]
- Chernoff AE, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, et al. (1995) A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol 154: 5492-9. [Crossref]
- Huhn RD, Radwanski E, O'Connell SM, Sturgill MG, Clarke L, et al (1996) Pharmacokinetics and immunomodulatory properties of intravenously administered recombinant human interleukin-10 in healthy volunteers. Blood 87: 699-705. [Crossref]
- van Deventer SJ, Elson CO, Fedorak RN (1997) Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology 113: 383-9. [Crossref]
- Asadullah K, Sterry W, Volk HD (2003) Interleukin-10 therapy--review of a new approach. Pharmacol Rev 55: 241-69. [Crossref]
- Nickoloff BJ, Fivenson DP, Kunkel SL, Strieter RM, Turka LA (1994) Keratinocyte interleukin-10 expression is upregulated in tape-stripped skin, poison ivy dermatitis, and Sezary syndrome, but not in psoriatic plaques. Clin Immunol Immunopathol 73: 63-8. [Crossref]
- Weiss E, Mamelak AJ, La Morgia S, Wang B, Feliciani C, et al. (2004) The role of interleukin 10 in the pathogenesis and potential treatment of skin diseases. J Am Acad Dermatol. 50: 657-75. [Crossref]
- Kaur S, Zilmer K, Leping V, Zilmer M (2014) Allergic contact dermatitis is associated with significant oxidative stress. Dermatol Res Pract 2014:415638.
- Kang K, Hammerberg C, Meunier L, Cooper KD (1994) CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J Immunol 153: 5256-64. [Crossref]
- Grundtner P, Gruber S, Murray SS, Vermeire S, Rutgeerts P, et al. (2009) The IL-10R1 S138G loss-of-function allele and ulcerative colitis. Genes Immun 10: 84-92. [Crossref]
- Li MC, He SH (2004) IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J Gastroenterol 10: 620-5. [Crossref]
- Liu XH, Ji QW, Huang Y, Zeng QT (2011) Th17 response promotes angiotensin II-induced atherosclerosis. Med Hypotheses 76: 593-5. [Crossref]
- Poredos P, Jezovnik MK (2011) In patients with idiopathic venous thrombosis, interleukin-10 is decreased and related to endothelial dysfunction. Heart Vessels 26: 596-602. [Crossref]
- Sikka G, Miller KL, Steppan J, Pandey D, Jung SM, et al. (2013) Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age. Exp Gerontol 48: 128-35. [Crossref]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA (2014) Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014: 149185.
- Iwakiri D (2014) Epstein-Barr Virus-Encoded RNAs: Key Molecules in Viral Pathogenesis. Cancers (Basel) 6: 1615-30.
- Zheng XY, Guan WJ, Mao C, Chen HF, Ding H, et al. (2014) Interleukin-10 promoter 1082/-819/-592 polymorphisms are associated with asthma susceptibility in Asians and atopic asthma: a meta-analysis. Lung 192: 65-73. [Crossref]
- Huhn RD, Radwanski E, Gallo J, Affrime MB, Sabo R, et al. (1997) Pharmacodynamics of subcutaneous recombinant human interleukin-10 in healthy volunteers. Clin Pharmacol Ther 62: 171-80. [Crossref]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683-765. [Crossref]
- Wissing KM, Morelon E, Legendre C, De Pauw L, LeBeaut A, et al. (1997) A pilot trial of recombinant human interleukin-10 in kidney transplant recipients receiving OKT3 induction therapy. Transplantation 64:999-1006. [Crossref]
- Pott GB, Sailer CA, Porat R, Peskind RL, Fuchs AC, et al (2007) Effect of a four-week course of interleukin-10 on cytokine production in a placebo-controlled study of HIV-1-infected subjects. Eur Cytokine Netw 18: 49-58. [Crossref]
- Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, et al (2009) IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res 104: e9-18. [Crossref]
- Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, et al. (2001) Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn's disease. Gut 49: 42-6. [Crossref]
- Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, et al (2000) Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology 119: 1473-82. [Crossref]
- Tilg H, van Montfrans C, van den Ende A, Kaser A, van Deventer SJ, et al. (2002) Treatment of Crohn's disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut 50: 191-5. [Crossref]
- Tilg H, Ulmer H, Kaser A, Weiss G (2002) Role of IL-10 for induction of anemia during inflammation. J Immunol 169: 2204-9. [Crossref]
- Döcke WD, Asadullah K, Belbe G, Ebeling M, Höflich C, et al. (2009) Comprehensive biomarker monitoring in cytokine therapy: heterogeneous, time-dependent, and persisting immune effects of interleukin-10 application in psoriasis. J Leukoc Biol 5: 582-93. [Crossref]
- Kimball AB, Kawamura T, Tejura K, Boss C, Hancox AR, et al. (2002) Clinical and immunologic assessment of patients with psoriasis in a randomized, double-blind, placebo-controlled trial using recombinant human interleukin 10. Arch Dermatol 138: 1341-6. [Crossref]
- Friedrich M, Döcke WD, Klein A, Philipp S, Volk HD, et al. (2002) Immunomodulation by interleukin-10 therapy decreases the incidence of relapse and prolongs the relapse-free interval in Psoriasis. J Invest Dermatol 118: 672-7. [Crossref]
- Gariboldi S, Palazzo M, Zanobbio L, Dusio GF, Mauro V, et al (2009) Low dose oral administration of cytokines for treatment of allergic asthma. Pulm Pharmacol Ther 22: 497-510. [Crossref]
- D’Amico L, Ruffini E, Ferracini R, Roato I (2012) Low Dose of IL-12 stimulates T Cell response in cultures of PBMCs derived from Non Small Cell Lung Cancer Patients. J Cancer Ther 3: 337-342.
- Cardani D, Dusio GF, Luchini P, Sciarabba M, Solimene U, et al (2013) Oral Administration of Interleukin-10 and Anti-IL-1 Antibody Ameliorates Experimental Intestinal Inflammation. Gastroenterol Res 6: 124-133.
- Radice E, Miranda V, Bellone G (2014) Low-doses of sequential-kinetic-activated interferon-gamma enhance the ex vivo cytotoxicity of peripheral blood natural killer cells from patients with early-stage colorectal cancer. A preliminary study Intern. Immunopharm 19: 66-73. [Crossref]
- Roberti ML, Ricottini L, Capponi A, Sclauzero E, Vicenti P, et al. (2014) Immunomodulating treatment with low dose Interleukin-4, Interleukin-10 and Interleukin-11 in psoriasis vulgaris. J Biol Regul Homeost Agents 28: 133-9. [Crossref]


