Abstract
Primary sclerosing cholangitis (PSC) is a chronic, progressive hepatobiliary disorder characterized by extensive fibrosis and stricturing of the intra- and/or extra-hepatic bile ducts. In the present study we tested the hypothesis that low phosphatylcholine (PC) levels in PSC bile permit toxic bile acid induced injury of biliary tract epithelial cells resulting in enhanced transforming growth factor-beta (TGF-β) expression and increased collagen synthesis by myofibroblasts. Thus, TGF-β mRNA expression was documented in bile duct epithelial cells exposed to varying concentrations of the toxic bile acid; glycochenodeoxycholic acid (GCDC) +/- PC. In these experiments, as well as in co-culture experiments where bile duct epithelial cells were cultured with peripheral blood mononuclear cells and myofibroblasts, TGF-β mRNA expression in all cell types remained unaltered. Moreover, collagen type Iα1 mRNA expression by myofibroblasts also remained unaltered. The results of this study do not support the hypothesis that PC deficiency contributes to toxic bile acid-induced bile duct injury and/or myofibroblast activation.
Key words
bile acids, primary sclerosing cholangitis, bile duct epithelial cells, myofibroblasts, phosphatylcholine, fibrosis, macrophages, cirrhosis
Introduction
Primary sclerosing cholangitis (PSC) is a chronic, progressive, fibrotic disorder of the hepatobiliary system that often progresses to cirrhosis [1]. The disease is more common in males, often in their third or fourth decades of life. Aside from endoscopic/surgical corrections of dominant biliary strictures, no specific treatments have been identified that favorably alter the course of the disease [2].
The pathogenesis of PSC remains unclear. One common hypothesis proposes that toxic bile acids induce biliary tract epithelial cell injury, resulting in the release of profibrotic cytokines such as transforming growth factor-beta (TGF-β) that activate neighbouring myofibroblasts and enhance collagen production [3-5]. Recent data indicating that PSC bile is deficient in phosphatylcholine (PC), a phospholipid released by hepatocytes into bile, which serves to protect epithelial cell membranes from the detergent effects of toxic bile acids, would support that hypothesis [6,7].
In the present study, we documented the effects of toxic bile acids on TGF-β mRNA expression in bile duct epithelial cells and collagen type Iα1mRNA expression in myofibroblasts in the presence and absence of exogenous PC.
Methods
Materials
KMBC bile duct epithelial cells were kindly provided by Dr. Gregory J. Gores at the Mayo Clinic School of Medicine while LX-2 myofibroblasts were donated by Dr. Scott L. Friedman at the Mount Sinai School of Medicine. All reagents for cell culture (Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), trypsin-EDTA, non-essential amino acids, glutamine and Trizol Reagent were purchased from Invitrogen (Burlington, ON). Polymerase chain reaction (PCR) primers were designed by the Oligo 5 computer software and synthesized by Invitrogen. SYBR® Safe DNA gel stain were purchased from Invitrogen (Invitrogen Canada Inc., Burlington, Canada). The iScript™ cDNA Synthesis Kit and iQ™ SYBR® Green Supermix were purchased from Bio-Rad Laboratories (Canada) Ltd. (Mississauga, Ontario). Co-culture plates were purchased from Corning (Corning Incorporated, NY, USA). The toxic bile acid glycochenodeoxycholate (GCDC), L-α-phosphatidylcholine (PC) and other chemicals were purchased from Sigma-Aldrich Co. (Oakville ON).
Cell culture
KMBC cells were cultured in DMEM supplemented with 110 mg/L sodium pyruvate, 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin. LX-2 cells were cultured in DMEM/F12 supplemented with 0.1 mmol/L non-essential amino acid, 2 mmol/L glutamine, 110 mg/L sodium pyruvate, 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin. All the cells were incubated at 37ºC in a humidified atmosphere of 5% CO2 and 95% air.
Co-culture of KMBC and LX-2 cells
KMBC and LX-2 cells were cultured in co-culture plates with inserts containing a 3.0-um porous membrane at 37ºC in a humidified atmosphere of 5% CO2 and 95% air. For each plate, 8 × 106 KMBC cells were cultured in the upper chamber and 8 × 106 LX-2 cells in the lower chamber. Culture medium was removed one day before treatment and cells were incubated in the medium described above. The indicated concentrations of GCDC were added into the upper chamber bath solution for 24h prior to cell harvesting.
Co-culture of KMBC peripheral blood mononuclear cells (PBMC) and LX-2 cells
KMBC, PBMC (derived from healthy donors) and LX-2 cells were cultured in co-culture plates with inserts containing a 3.0-um porous membrane at 37ºC in a humidified atmosphere of 5% CO2 and 95% air. For each well, 5 × 106 KMBC and 3 × 106 PBMC cells were cultured in the upper chamber and 8 × 106 LX-2 cells in the lower chamber. Culture medium was removed one day before GCDC exposure and the cells incubated in the medium described above.
Extraction of RNA
TRIzol® reagent was employed to extract total RNA from cells according to the manufacturer’s instructions. Briefly, cells were dissolved in 1 ml TRIzol® reagent and incubated at room temperature for 5 min to permit complete dissociation of the nucleoprotein complex. After adding 0.2 ml chloroform, tubes were shaken vigorously by hand for 15 sec, and incubated for 2 min at room temperature. After centrifuging the sample at 12,000xg for 15 min at 4ºC, the mixture separated into a lower red phenol-chloroform phase, a white interphase, and a clear upper aqueous phase. The aqueous phase which contained RNA was transferred into new Eppendorf tubes and RNA was precipitated using 0.5 ml isopropyl alcohol. Samples were incubated at room temperature for 10 min and then centrifuged at 12,000xg for 10 min at 4ºC. RNA pellets were washed with 1 ml 75% ice-cold ethanol and centrifuged at 7,500xg for 5 min at 4ºC. After being air-dried for 10 min, RNA pellets were resuspended in DEPC water. RNA concentration was assessed by Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, Vermont, USA). Absorbance of each diluted RNA sample was measured at wavelengths 260 nm and 280 nm. RNA was then stored at -80ºC until used in further experiments.
Reverse transcriptase PCR (RT-PCR)
The first strand cDNA was synthesized by the iScript™ cDNA Synthesis Kit as per the manufacturer’s manual. PCR was performed using the iQ™ SYBR® Green Supermix and the oligonucleotides synthesized by Invitrogen. The specific primers for the present study were designed with the respective sequences from GenBank by Oligo 7 program and listed in Table 1. PCR amplification was carried out by applying 28-30 cycles comprising: denaturation at 94ºC for 1 minute, different annealing temperatures for 30 seconds, elongation at 72ºC for 2 minutes, followed by a final elongation at 72ºC for 8 minutes using a MiniOpticon™ Real-Time PCR Detection System (Bio-Rad Laboratories (Canada) Ltd. Mississauga, Ontario). PCR products were also analyzed by electrophoresis on a 1.0% agarose gel. Identity of PCR products was confirmed by DNA sequencing at the DNA sequencing facility of the Manitoba Institute of Cell Biology.
Genes |
|
Primers |
Tm
(ºC) |
Size
(bp) |
TGF-β1 |
sense |
5’-TACCTGAACCCGTGTTGCTCT-3’ |
59.5 |
210 |
|
anti-sense |
5’-TTTCCCCTCCACGGCTCAACC-3’ |
|
|
Collagen |
sense |
5’-CTCCTGGCAAAGATGGACTCA-3’ |
60.8 |
544 |
|
anti-sense |
5’-ATGCTCTCGCCGAACCAGA-3’ |
|
|
Β-actin |
sense |
5’-GCACCACACCTTCTACAATG-3’ |
60 |
838 |
|
anti-sense |
5’-TGCTTGCTGATCCACATCTGS-3’ |
|
|
Table 1. Primers and conditions of polymerase chain reaction
Results
In the first series of experiments, KMBC cells were exposed to varying concentrations of GCDC (5-500 µM) alone or 50 µM GCDC + PC (100 µM). As shown in Figure 1, TGF-β mRNA expression in KMBC cells remained unaltered in the presence of GCDC and GCDC + PC.
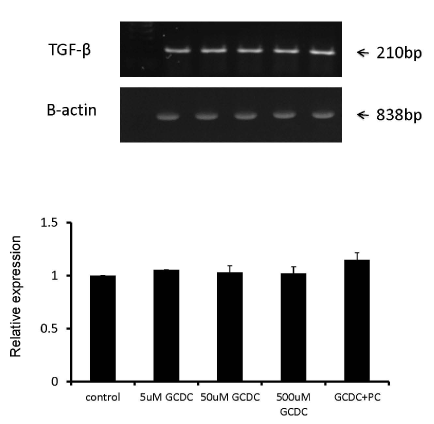
Figure 1. No induction or reduction of TGF-β mRNA expression after GCDC +/- PC treatment in KMBC cells. KMBC cells were treated by 5uM, 50uM, 500uM of GCDC alone or 50uM GCDC with 100uM PC. The mRNA abundances of TGF-β in KMBC cells were examined and remained unaltered in the presence of GCDC and GCDC + PC.
Because an autocrine loop has been described for TGF-β and myofibroblasts (8), we also exposed LX-2 cells to GCDC (5-500 µM) alone and GCDC (50 µM) + PC (100 µM) but again, TGF-β mRNA expression remained unaltered (Figure 2).
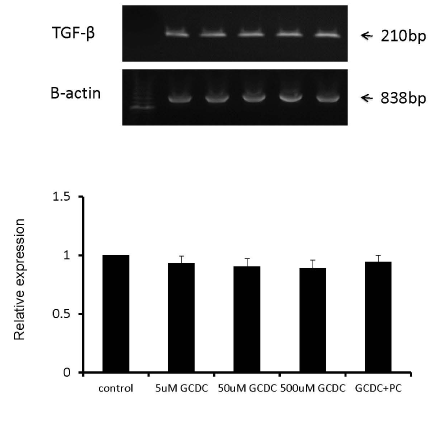
Figure 2. There was no alteration of TGF-β mRNA expression after GCDC +/- PC treatment in LX-2 cells. LX-2 cells were treated by 5uM, 50uM, 500uM of GCDC alone or 50uM GCDC with 100uM PC. The mRNA abundances of TGF-β in LX-2 cells were examined and remained unaltered in the presence of GCDC and GCDC + PC.
To exclude the possibility that GCDC exposure results in the release of pro-fibrotic cytokines other than TGF-β, which in turn stimulate LX-2 fibrogenic activity, we cultured GCDC (50 µM) +/- PC (100 µM) exposed KMBC cells in the upper chamber and LX-2 in the lower chamber of co-culture plates. Collagen Iα1 mRNA expression by LX-2 cells was then determined by RT-PCR. As shown in Figure 3, collagen mRNA expression remained unaltered in these experiments.
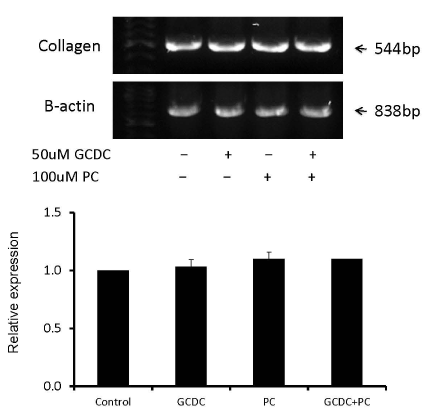
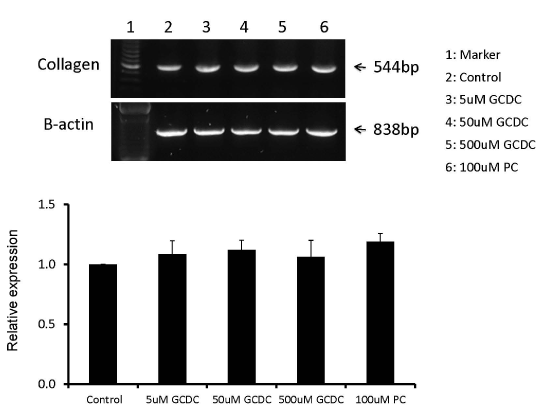
Figure 3. No induction or reduction of collagen mRNA expression in LX-2 cells in the lower chamber after GCDC +/- PC treatment of KMBC cells in the upper chamber. KMBC cells were treated by 50uM of GCDC, 100uM of PC and 50uM GCDC with 100uM PC. The mRNA abundances of collagen in LX-2 cells were examined and there were no significant differences between GCDC, PC and GCDC+PC treatments.
Finally, to determine whether macrophage activation (resulting from epithelial cell damage following exposure to toxic bile acids) is required to stimulate myofibroblast activity, KMBC cells exposed to GCDC (5-500 µM) +/- PC (100 µM) were co-cultured with PBMCs in the upper chamber and LX-2 cells in the lower chamber. Once again, collagen Iα1 mRNA expression in LX-2 cells remained unaltered in these experiments (Figure 4).
 within PSC bile; increased concentrations of toxic bile acids in cholestatic livers; high levels of TGF-β mRNA and protein expression in PSC liver and improved outcomes with TGF-β lowering agents in animal models of PSC [9-12]. However, the results of the present study are not supportive of the hypothesis that PC deficiency in PSC bile permits toxic bile acid-induced biliary tract epithelial injury which in turn, results in enhanced TGF-β expression (by bile duct epithelial cells and/or tissue macrophages) resulting in activation of adjacent myofibroblasts. The results also argue against toxic bile acids directly altering TGF-β mRNA expression and collagen synthesis by myofibroblasts. Finally, the incorporation of co-culture experiments served to exclude the possibility that cytokines other than TGF-β (and/or the presence of macrophages) are required for toxic bile acid-induced activation of myofibroblasts.</p>
<p>Notwithstanding the above findings, there are a number of limitations to the study that warrant emphasis. First, the cells employed included bile duct epithelial and myofibroblast cell lines rather than primary cells derived from human livers. Whether these cell lines are less sensitive to the toxic effects of bile acids and pro-fibrogenic cytokine stimulation than primary cells remains to be determined. Second, the concentration range of toxic bile acids employed was derived from previous reports documenting concentrations of GCDC in human blood [13]. Whether higher concentrations are present in human bile and in particular, the bile of PSC patients, is unclear. Third, the co-culture experiments physically separated bile duct epithelial and peripheral blood mononuclear cells from myofibroblasts thereby preventing cell-cell contact and possible intercellular communication. Fourth, perhaps longer periods of cell exposure to toxic bile acids were required to induce bile duct epithelial cell injury. However, the rapid biochemical and histologic changes associated with acute bile duct ligation models argue against that possibility [14]. Fifth, it should be noted that PBMCs consist of peripheral blood monocytes and not tissue macrophages. Perhaps the additional features of the latter cell population are essential for the expression and release of pro-fibrogenic cytokines in this setting [15]. Finally, we did not explore the possibility that restoring bile PC concentrations to normal levels may favorably alter the course of PSC by means other than protecting biliary tract epithelial cells from toxic bile acid-induced injury.</p>
<p>In conclusion, the results of this study do not support the hypothesis that PC deficiency permits toxic bile acid-induced injury of biliary tract epithelial cells and subsequent activation of adjacent myofibroblasts, resulting in the enhanced fibrosis seen in PSC. Thus, at this time, restoration of low PC levels to normal values in PSC bile does not appear to be a worthwhile therapeutic approach to the treatment of PSC.</p>
<h2>Acknowledgements</h2>
<p>The authors wish to thank Dr. G. Hatch for his guidance and Ms R. Vizniak for her prompt and accurate typing of the manuscript.</p>
<h2>References</h2>
<ol>
<li>Lee YM, Kaplan MM (1995) Primary sclerosing cholangitis. <em>N Engl J Med</em> 332: 924-933. [<a href=)
Crossref]
Karlsen TH, Schrumpf E, Boberg KM (2010) Primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol 24: 655-666. [Crossref]
Palmer RH (1972) Bile acids, liver injury, and liver disease. Arch Intern Med 130: 606-617. [Crossref]
Choi HS, Savard CE, Choi JW, Kuver R, Lee SP (2007) Paclitaxel interrupts TGF-beta1 signaling between gallbladder epithelial cells and myofibroblasts. J Surg Res 141: 183-191. [Crossref]
Krones E, Graziadei I, Trauner M, Fickert P (2012) Evolving concepts in primary sclerosing cholangitis. Liver Int 32: 352-369. [Crossref]
Khan SA, Cox IJ, Thillainayagam AV, Bansi DS, Thomas HC, Taylor-Robinson SD (2005) Proton and phosphorus-31 nuclear magnetic resonance spectroscopy of human bile in hepatopancreaticobiliary cancer. Eur J Gastroenterol Hepatol 17: 733-738. [Crossref]
Dial EJ, Rooijakkers SH, Darling RL, Romero JJ, Lichtenberger LM (2008) Role of phosphatidylcholine saturation in preventing bile salt toxicity to gastrointestinal epithelia and membranes. J Gastroenterol Hepatol 23: 430-436. [Crossref]
Biernacka A, Dobaczewski M, Frangogiannis NG (2011) TGF-β signaling in fibrosis. Growth Factors 29: 196-202. [Crossref]
Trauner M, Fickert P, Wagner M (2007) MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis 27: 77-98. [Crossref]
Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, et al. (2012) Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int 32: 58-69. [Crossref]
Michel K, Roth S, Trautwein C, Gong W, Flemming P, Gressner AM (1998) Analysis of the expression pattern of the latent transforming growth factor beta binding protein isoforms in normal and diseased human liver reveals a new splice variant missing the proteinase-sensitive hinge region. Hepatology 27: 1592-1599. [Crossref]
Strack I, Schulte S, Varnholt H, Schievenbusch S, Töx U, et al. (2011) β-Adrenoceptor blockade in sclerosing cholangitis of Mdr2 knockout mice: antifibrotic effects in a model of nonsinusoidal fibrosis. Lab Invest 91: 252-261. [Crossref]
Adachi Y, Nanno T, Itoh T, Kurumi Y, Yamazaki K, et al. (1988) Determination of individual serum bile acids in chronic liver diseases: fasting levels and results of oral chenodeoxycholic acid tolerance test. Gastroenterol Jpn 23: 401-407. [Crossref]
Rodríguez-Garay EA (2003) Cholestasis: human disease and experimental animal models. Ann Hepatol 2: 150-158. [Crossref]
Cameron RG, Blendis LM, Neuman MG (2001) Accumulation of macrophages in primary sclerosing cholangitis. Clin Biochem 34: 195-201. [Crossref]