Catheter associated urinary tract infections (CAUTI) continue to be the most challenging of all hospital and institutional acquired conditions. According to the Centers for Disease Control and Infection Prevention urinary tract infections (UTIs) are the most common type of healthcare-associated infection reported to the National Healthcare Safety Network (NHSN) [1]. Urinary infections are the source for more than 50% of all infections in long term care facilities and more than 40% of all hospital acquired infections [2]. Despite extreme efforts, including the off-label use of topical drugs, the CAUTI rate world-wide continues to rise. This is only further complicated by the emergence of multi-drug resistant organisms (MDROs). Considerable personnel time, direct and indirect costs are expended by health care institutions to reduce the rate of hospital acquired infections, especially those that occur in patients with signs and symptoms referable to the urinary tract. UTIs are classified as either uncomplicated or complicated infections and treatment often differs based on this classification. However, all nosocomial and catheter associated urinary tract infections are considered complicated. Nosocomial infections are further complicated by advanced patient age and multiple comorbidities [3]. The likelihood of treatment failure and serious complications, particularly the development of antimicrobial resistance, is more common in CAUTI. Multiple drug resistant organisms including Carbapenem Resistant Enterobacteriaceae (CRE), in particular, an E coli isolate, adds to the complex challenge as many ICU patients with indwelling catheters experience fecal incontinence [4]. The removal of macroscopic debris during cleansing of incontinent patients is not sufficient to prevent infections. Therefore, topical antiseptic solutions and cleansers should have proven efficacy demonstrating the ability to decolonize these resistant organisms on human skin and mucosa without disrupting the normal host immunity, while preserving mucosal integrity and the microbiome [5,6].
The diligence required to maintain an indwelling catheter is reported as a main contributor to whether infection develops. The demands on nursing and ancillary staff can prove to prohibit necessary catheter maintenance steps to prevent CAUTI. Effective perineal protocols require nursing management, quality and supply chain acceptance and commitment for each institution. To date, only two methodologies have consistently proven to be effective in reducing CAUTI rates; implementing closed catheter systems and early removal of the catheter [7]. Scheduled cleansing of the catheter and urethral meatus, referred to as catheter care and maintenance, deserves scrutiny when designing a program to prevent CAUTI. Multiple studies have evaluated the effectiveness of antiseptic cleansers, ointments, or creams. Dr. Mikel Gray sites work by Dr. Koskeroglu et al evaluating the effectiveness of 4 separate protocols for urethral meatus care in ICU patients. The techniques employed were as follows: (1) cleansing plus once daily application of a 9% povidone- iodine solution, (2) cleansing plus twice daily application of a 9% povidone-iodine solution, (3) once-daily cleansing using a 4% chlorhexidine gluconate, (4) twice daily cleansing with a 4% chlorhexidine gluconate, and (5) a control group. None of the protocols that employed antiseptic solutions proved more effective for preventing CAUTI, bacteriuria, or bacterial colonization at the urethral meatus than routine cleansing alone [8].
In June 2016, The National Comprehensive Unit-Based Safety Program which is funded by the Agency for Healthcare Research and Quality reported on their 5 year outcomes of their national prevention program initiative which was implemented in 926 units across hospitals in 32 states. This included 60% non-ICU based units and 40% ICU based units. Their conclusions were that only non-ICU based units benefited from participation in their program with a 14% decrease in CAUTIs over a 4-year period. ICU based units saw an alarming 9% increase in CAUTI in the same 4-year period. Importantly, their protocol did not suggest the implementation of any products that have been suggested to reduce CAUTI rates [9].
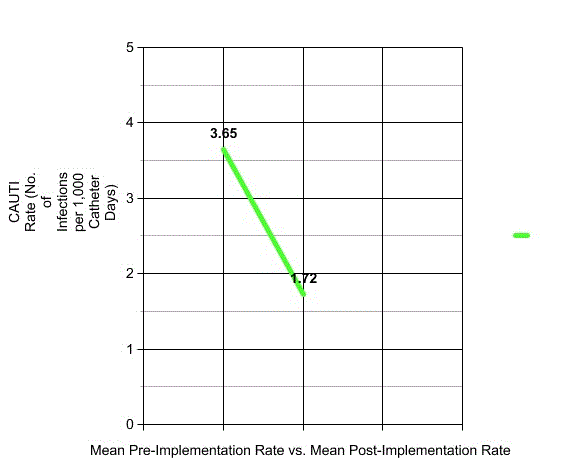
Figure 1. Mean CAUTI Rate Reduction with Theraworx Use.
Table 1: Results by Hospital.
Site |
CAUTI Rate Pre-Intervention Period
(per 1,000 catheter days) |
Pre-Intervention Period Start Date |
Pre-Intervention Period End Date |
Pre-Intervention Period
in Months |
CAUTI Rates Post-Theraworx Intervention
(per 1,000 catheter days) |
Post-Theraworx Intervention Start Date
|
End Date of Theraworx Intervention Data |
Post-Theraworx Intervention Period
in Months |
Percent Change |
University Hospital
(San Antonio, Tex.) |
3.16 |
1/2015 |
3/2015 |
2 |
2.45 |
4/2015 |
6/2016 |
14 |
-22.47% |
Baylor Scott & White Medical Center – Centennial (Frisco, Tex.) |
1.84 |
11/2013 |
10/2015 |
23 |
0 |
11/2015 |
6/2016 |
7 |
-100.00% |
South Miami Hospital/Baptist Health South Florida
(South Miami, Fla.) |
3.04 |
1/2013 |
12/2014 |
23 |
0.51 |
12/2014 |
6/2016 |
18 |
-83.22% |
First Health [TK – need to know which of the four First Health hospitals this is] |
1.25 |
1/2012 |
12/2013 |
23 |
0.65 |
1/2014 |
12/2015 |
23 |
-48.00% |
Mercy Hospital Springfield
(Springfield, Mo.) |
2.34 |
8/2013 |
7/2015 |
23 |
1.4 |
8/2015 |
7/2016 |
11 |
-40.17% |
St. Joseph Medical Center
(Towson, Md.) |
3.34 |
7/2013 |
10/2015 |
27 |
0 |
12/2015 |
6/2016 |
5 |
-100.00% |
Little Company of Mary Hospital
(Evergreen Park, Ill.) |
2.93 |
1/2012 |
12/2014 |
35 |
1.17 |
1/2015 |
9/2016 |
20 |
-60.07% |
Regional Medical Center at Memphis, Memphis, Tenn. |
11.3 |
N/A |
N/A |
- |
7.55 |
N/A |
N/A |
- |
-33.19% |
Large Florida university hospital
(NON-COMPLIANT SITE) |
2.87 |
1/2015 |
8/2015 |
7 |
3.74 |
10/2015 |
9/2016 |
11 |
30.31% |
Large
Kansas City hospital
(NON-COMPLIANT SITE) |
1.2 |
1/2013 |
5/2015 |
28 |
1.2 |
5/2015 |
1/2016 |
8 |
0.00% |
Total for compliant sites |
3.65 |
|
|
22.29 |
1.72 |
|
|
14 |
-52.88% |
Recently, the CDC conducted the CUSP study to determine the effectiveness of soap and water cleansing in reducing CAUTI in more than 600 hospitals and found that this contributed to only a 1% reduction in ICU setting and 14% reduction on general medical floors [10]. This demonstrates the soap and water care is beneficial as an initial step in a protocol to reduce macroscopic debris and prepare the perineum for application of other agents that may be additive in their effect on reducing CAUTI. Less than expected results from all products and methodology point to the need for quality improvement oversight and implementation for evidenced based practice guidelines including comprehensive education across all departments [11-14]
Ten hospitals which have implemented the Theraworx skin care system protocol for an extended period of greater than 20 months and which demonstrated excellent quality in providing confirmed data for the 40+ month period were queried for their outcome data regarding CAUTIs. These institutions applied Theraworx in a variety of care settings including high-risk neurological, cardiovascular and trauma intensive care units. Many patients were incontinent of urine, feces or both in these settings. Theraworx was used in all of its available forms: foam, spray or moisture-impregnated cloths. The clinical protocol was to apply Theraworx to the meatus and surrounding tissue, prior to insertion of a catheter, to establish a zone of inhibition, then reapply the product TID and after each incident of fecal incontinence as a maintenance intervention. The hospitals were asked to provide insertion and maintenance details on utilization, pre-and post-implementation CAUTI rates as reported to the National Healthcare Safety Network. The hospitals reported their outcome data and were included in our retrospective analysis. Compliance to the protocol was assessed at each institution. Post implementation results were then compared to an extended pre-implementation period with analogous patient populations. CAUTI were reported per 1,000 catheter days consistent with the CDC reporting nomenclature and AHRQ [9].
Ten of the hospitals from which data was requested provided both pre- and post-intervention data. Eight of the ten reporting hospitals complied with recommended clinical protocols.
The average pre-intervention period for these eight hospitals was 22.9 months. The average post-intervention period was 14 months. All eight hospitals reported that Theraworx use markedly reduced their CAUTI rate. The reductions ranged from 22.47% (UHS-San Antonio in San Antonio, Tex.) to 100% at two hospitals (Centennial Hospital, in Frisco, Texas and Peace health St. Joseph Medical Center, in Bellingham, Washington). CAUTIs were completely eliminated at the latter two sites. The mean pre-intervention CAUTI rate for the eight compliant hospitals was 3.65/1,000 catheter days. The mean post-intervention CAUTI rate for those same hospitals was 1.72/1,000 catheter days, a difference of 1.93/1,000 catheter days. Therefore, the mean change in CAUTI rate was a reduction of 52.88% for compliant institutions.
Evaluation of the two hospitals that acknowledged that proper protocols for Theraworx use were not consistently followed during the study period revealed troubling data. One of these hospitals reported no change in CAUTI rates; while the other reported a 30.31% increase.
The Theraworx skin care system (Avadim Technologies, Asheville, NC), is a non-toxic topical skin and mucosal membrane application that was associated with a significant reduction in CAUTIs over a period of 20 months or more of implementation at hospitals, across the United States, in a myriad of high risk ICU patient care settings. Upon implementation of the Theraworx protocol, CAUTI rates decreased between 22.47% and 100% (complete elimination of CAUTIs) in 8 of the 10 institutions which remained compliant. This represented a mean overall decrease in CAUTIs of more than 53% among responding hospitals that followed the recommended clinical protocols. It is also notable that the 2 noncompliant sites were the only sites that did not achieve a substantial improvement in their CAUTI rates and in one instance CAUTI rates actually increased.
This study encompassed several different acute care settings including high risk hospital units such as neurological, cardiovascular and trauma ICUs. Further, many of the patients treated with the novel skin formulation were at an increased risk for urinary tract infection due to their concomitant medical comorbidities and urinary/fecal incontinence.
The proposed mechanism of action is reduction in the pH, and therefore optimization, of the stratum corneum layer of the skin, urethral meatus and vaginal mucosa. By reducing the pH to 5-5.5 the stratum corneum is able to function at its maximal immune and barrier capacity. Further, at the lower pH the normal dermal microbiome is preserved thus reducing the presence of more virulent bacteria, fungi or viruses. This paradigm shift is an exciting approach to the prevention of infections and additional attempts at the reduction of the further development of multi-drug resistant organisms by reducing antibiotic use.
Our study has limitations due to its non-randomized and retrospective nature. It is very difficult to get multiple institutions and staff individuals to adhere to a protocol for catheter insertion and maintenance, especially in the ICU setting given the complexity of the patient’s disease state. The inclusion of the non-compliant sites is evidence to this and there lack of improvement or increase in CAUTI rate reflects the need for implementation and adherence to such protocols.
Results of this aggregated data set suggest that patients at hospitals with CAUTIs may benefit from use of Theraworx skin formulation at catheter insertion and for catheter maintenance. This approach may be especially appropriate for institutions that are failing to meet their own or national CAUTI benchmarks and suffering financial penalties for these critical HAIs. We encourage institutions to consider including the Theraworx protocol into their CAUTI clinical pathway to potentially achieve additional reduction in their CAUTI rates.
- Centers for Disease Control and Prevention (CDC): National Healthcare Safety Network (NHSN) Report, Catheter- associated Urinary Tract Infections (CAUTI).
- Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, et al. (2010) Diagnosis, prevention and treatment of catheter-associated urinary tract infection in adults; 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50: 625-663. [Crossref]
- Nicolle LE (2001) A practical guide to antimicrobial management of complicated urinary tract infection, Drugs Aging 18: 243-54.
- Brennan BM, Coyle JR, Marchaim D, Pogue JM, Boehme M, et al. (2014) Statewide surveillance of carbapenem-resistant Enterobacteriaceae in Michigan. Infection Control Hosp Epidemiol 35: 342-349. [Crossref]
- Matsumoto T, Sakumoto M, Takahashi K, Kumazawa J (1997) Prevention of catheter associated urinary tract infection by meatal disinfection. Dermatology 195: 73-77. [Crossref]
- Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, et al. (1981) Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am J Med 70:655-658. [Crossref]
- Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, et al. (2013) Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf [Crossref]
- Gray M (2010) Reducing Catheter-Associated Urinary Tract Infection in the Critical Care Unit. American Association of Critical–Care Nurses 21: 247-257. [Crossref]
- Saint S, Greene MT, Krein SL, Rogers MA, Ratz D, et al. (2016) A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. N Engl J Med 374: 2111-2119. [Crossref]
- Castalucci M (2016) National Study Shows Decreased Rate of Catheter Associated UTI’s. Modern Healthcare.
2021 Copyright OAT. All rights reserv
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA (2009) Healthcare Infection Control Practices Advisory Committee (HICPAC): guideline for prevention of catheter-associated urinary tract infections.
- Pratt RJ, Pellowe C, Loveday HP, Robinson N, Smith GW (2001) Epic Guideline Development Team. Guidelines for preventing infections associated with the insertion and maintenance of short-term indwelling urethral catheters in acute care. J Hosp Infect 47: S39–S46. [Crossref]
- Pratt RJ, Pellowe CM, Wilson JA (2007) Epic 2: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 65: S1-S64. [Crossref]
- Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, et al. (2014) Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf 23: 277-289. [Crossref]

