Transplantation of alginate-encapsulated islets has the potential to treat patients suffering from type I diabetes, a condition characterized by an autoimmune attack against insulin-secreting β-cells. However, there are multiple challenges associated with this procedure, all of which must be adequately addressed prior to translation from trials in small animal and non-human primate models to human clinical trials. The lack of standardization of significant parameters of encapsulation device design and manufacture (i.e. purification protocols, surface-modification grafting techniques, alginate composition modifications) between labs is yet another obstacle that must be overcome before a clinically effective and applicable protocol for encapsulating islets can be implemented. The response of these microcapsules to long-term culture at physiological conditions has not been studied. In this study, alginate microcapsules were polymerized with either barium or calcium, to evaluate their morphological responses in order to choose an optimal gelling ion for future utilization in islet transplantation. We used two variation of alginates: high mannuronate (UPLVM) and high guluronate (UPLVG) alginate. Both barium and calcium crosslinked encapsulated islets successfully restore glucose control in diabetic athymic nude mice. Because the capsule size change overtime is significant, it’s important to take this into account when choosing which alginate and which gelling ion will be used to make the capsules, to make sure that the end size is as intended to minimize hypoxic cores of the encapsulated islets, instead of just relying on capsule size immediately after polymerization. Our results showed that compared to the standard calcium ions, barium ions used for crosslinking alginate resulted in smaller size change of UPLVM capsules, thus maintaining the porosity and permeability.
alginate, polymerization, islet encapsulation, isotropic shrinking, isotropic swelling
Type 1 diabetes is characterized by the autoimmune mediated destruction of β-cells in the pancreas. Since these cells produce the hormone insulin, which is required to maintain control over blood glucose level by inducing uptake of glucose from the blood into cells, lack of insulin in type 1 diabetes leads to high blood glucose levels, and susceptibility to tissue damage, amputation, blindness, and death [1, 2]. Over the last decade, human islet transplantation has emerged as a viable alternative to conventional management strategies to treat type 1 diabetes. With appropriate immunosuppression regimens, patients that are able to remain insulin independent for up to 8 years post-transplant [3, 4]. However, immunosuppression itself has several side effects, including susceptibility to infection, and toxicity to the transplanted islets. In the end, this leads to the search of technology to protect transplanted islets against rejection while minimizing or eliminating the requirement for immunosuppression [5].
Encapsulation of islets within biocompatible hydrogels is a solution proposed by biomedical engineers to address this uniquely frustrating issue. Cell encapsulation involves enveloping cells in a selectively permeable biocompatible matrix that allows for the diffusion of oxygen and nutrients but is able to effectively prevent immune cells and antibodies from reaching the graft, thus delaying rejection [6]. Encapsulation can be employed as a platform to deliver localized immunosuppression at the transplant site, thus avoiding the adverse effects of chronic systemic immunosuppression and can also be used to deliver nutrients and biological agents that will enhance islet survival and function after transplantation into patients [7, 8]. While encapsulation has several advantages over conventional islet transplantation, several roadblocks currently prevent translation of results from small animal and primate trials to human trials. Alginate pore size is a crucial parameter that aids in the protection of islets from host immune recognition, while allowing insulin, oxygen, and other micronutrients to diffuse through the capsules. Islet encapsulation within alginate hydrogels is advantageous because it prevents direct contact between the encapsulated islets and the host immune system while significantly reducing the need for chronic systemic immunosuppression [9, 10]. Although several studies have demonstrated the utility of alginate microencapsulation in islet and stem cell transplantation [11], the response of these microcapsules to long-term culture at physiological conditions has hitherto not been evaluated. It is believed that alginate microcapsules exhibit isotropic shrinking at high culture temperatures and isotropic swelling in the presence of sodium ions. In this study, we evaluated various gelling ions and alginates to study their responses at specific temperatures and at physiological sodium ion concentrations to develop strategies to mitigate sodium ion-induced isotropic swelling of the alginate capsules.
Effect of Gelling Ion Type and Concentration on Microcapsule Size
Aqueous solutions of ultra-pure low viscosity mannuronate (UPLVM, NovaMatrix® PRONOVA™) and ultra-pure low viscosity guluronate (UPLVG, NovaMatrix® PRONOVA™) at 2.5% w/v were used to generate alginate microcapsules using a compressed air-driven electrostatic encapsulator (Nisco Engineering AG). Standard settings of 4 psi (pressure), 80 rpm (agitator speed), 30 mm (needle height) and 25G (needle height) were used. Three different divalent ion solutions were compared: 20mM BaCl2, 50mM BaCl2, and 120mM CaCl2 (n=3, performed in triplicate). After each experiment, a minimum 100 microcapsules were imaged using an inverted bright field microscope after 30 min of crosslinking. Microcapsules were then stored in a buffered solution containing proteins and ions at physiological concentrations at 37°C. (Table 1).
Table 1. Component, concentration, and ions for physiological solution.
Physiological solution was made to simulate the environment in which the capsules will be subjected to in the body.
Component |
Concentration |
Na+ |
139 mM |
Ca2+ |
1.2 mM |
Glucose |
5.6 mM |
Urea |
5.5 mM |
Albumin |
1.4 mM |
Protein |
2.2 mM |
PO43- |
1.02 mM |
pH |
7.4 |
After 1, 7, and 14 days of incubation, groups of minimum of 100 microcapsules were quantified for changes in diameter. Images obtained as described were processed using a batch-processing algorithm on Image J (NIH) to calculate microcapsule size. All results are expressed as mean± s.e.m. A Mann-Whitney Test was performed to analyze whether the change in microcapsule size was statistically significant (p<0.05).
Effect of Gelling Ion Type and Concentration after in vivo Transplant
Aqueous solutions of ultra-pure low viscosity mannuronate (UPLVM, NovaMatrix® PRONOVA™) and ultra-pure low viscosity guluronate (UPLVG, NovaMatrix® PRONOVA™) at 2.5% w/v were used to generate alginate microcapsules using a compressed air-driven electrostatic encapsulator (Nisco Engineering AG). Standard settings of 4 psi (pressure), 80 rpm (agitator speed), 30 mm (needle height) and 25G (needle height) were used. Two different divalent ion solutions were used to crosslink the alginate capsules for 30 minutes: 50mM BaCl2, and 120mM CaCl2. The capsules were then incubated overnight in 37°C 5% CO2 apparatus in islet media, CMRL 1066 (Cellgro) with 10% newborn calf serum (Sigma Aldrich), supplemented with 5 mM BaCl2 or CaCl2, respective to the original crosslinking ion. After the overnight incubation, a minimum of 100 capsules were imaged for size measurement.
The capsules were then transplanted into nondiabetic athymic nude (n=5) or CD1 (n=5) mice, with 1000 capsules transplanted intraperitoneally. After 14 days, the mice were euthanized, the peritoneal cavity was flushed using normal saline, and the capsule diameter was measured by imaging and ImageJ analysis. All animal procedures were conducted under University of California Irvine, Institutional Animal Care and Use Committee approved protocol #2008-2850.
Effect of Gelling Ion Type and Concentration after in vivo Transplant of Encapsulated Islets in Diabetic Mice
Islets were isolated using standard collagenase digestion and gradient purification from Sprague Dawley rats [12]. Aqueous solutions of ultra-pure low viscosity mannuronate (UPLVM, NovaMatrix® PRONOVA™) at 2.5% w/v were used to generate alginate microcapsules using a compressed air-driven electrostatic encapsulator (Nisco Engineering AG). Standard settings of 4 psi (pressure), 80 rpm (agitator speed), 30 mm (needle height) and 25G (needle height) were used. Two different divalent ion solutions were used to crosslink the alginate capsules for 30 minutes: 50mM BaCl2 and 120mM CaCl2. The capsules were then incubated overnight in 37°C 5% CO2 apparatus in islet media, CMRL 1066 (Cellgro) with 10% newborn calf serum (Sigma Aldrich), supplemented with 5 mM BaCl2 or CaCl2, respective to the original crosslinking ion.
Immune competent CD1 mice were made diabetic using streptozotocin, 150 mg/kg, injected intraperitoneally under anesthesia [13]. After 2 consecutive days of blood glucose >350 mg/dL, the diabetic mice received transplant of the encapsulated islets (n=5 per gelling ion type), with 2000 encapsulated islets transplanted intraperitoneally per mouse. After 14 days, the mice were euthanized, the peritoneal cavity was flushed using normal saline, and the islet viability was measured by fluorescein diacetate/propidium iodide fluorescent staining [14]. All animal procedures were conducted under University of California Irvine, Institutional Animal Care and Use Committee approved protocol #2008-2823 (for islet isolation from Sprague Dawley rats) and 2008-2850 (for transplantation).
Statistical Analysis
The experiments were repeated three times to ensure that the results obtained were consistent. A Mann-Whitney Test was performed to analyze whether the change in microcapsule size was statistically significant (p<0.05). For transplant studies, a one way ANOVA followed by a post-hoc Tukey HSD test was used to determine statistical significance with a p<0.05 considered statistically significant. All data was reported as mean ± s.e.m.
Effect of Gelling Ion Type and Concentration on Microcapsule Morphology
After 1 day of incubation in physiological buffer, all barium-gelled capsules shrink in size, whereas calcium-gelled capsules shrink in size except for calcium-gelled UPLVM capsules, which showed significant increase in diameter. In all groups, capsules polymerized using 50 mM BaCl2 showed the least amount of change in diameter. This pattern continues all the way to 14-days post incubation (Figure 1). In UPLVM capsules (Figure 1A), there is a significant difference between size changes when gelling with 20 compared 50 mM BaCl2. In UPLVG capsules (Figure 1B), this difference is only significant until 7 days after incubation in physiological solution.
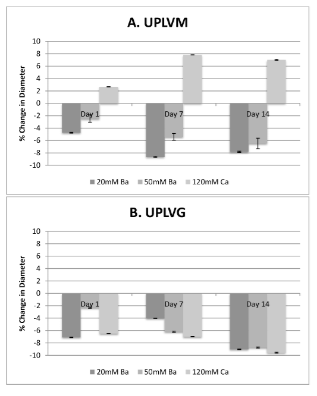
Figure 1. Gelling ion variations and their effect on size after in vitro incubation in physiological solution.
UPLVM or UPLVG capsules, gelled in 20 mM or 50 mM BaCl2, or 120 mM CaCl2, was incubated in physiological solution for up to 14 days in 37°C 5% CO2 incubator. Size of the capsules were measured by image analysis of a minimum of 100 capsules at 1, 7, and 14 days post incubation. All results are shown as mean ± SEM.
Effect of Gelling Ion Type and Concentration after in vivo Transplant
Barium gelled UPLVM capsules show a decrease in diameter when compared to calcium gelled UPLVM capsules, which showed an increase in diameter after transplantation (Figure 2A). In comparison, UPLVG capsules gelled with barium had a larger decrease in diameter, whereas UPLVG capsules gelled with calcium had a decrease in diameter when transplanted into CD1 (immunocompetent) mice but not when transplanted into athymic nude (immunodeficient) mice (Figure 2B). Our results showed that barium gelled UPLVM capsules had significantly less change in capsule size when compared to calcium gelling or UPLVG alginate (Figure 2).
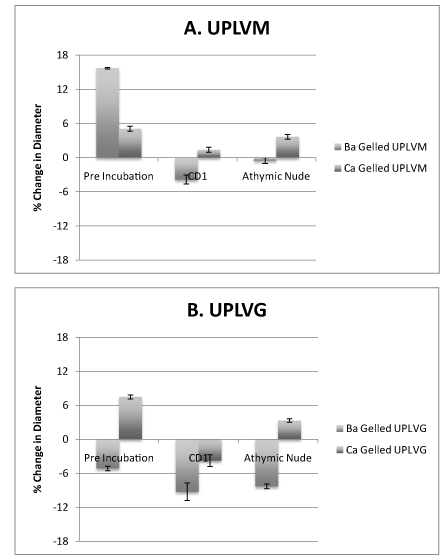
Figure 2. Gelling ion variations and their effect on size after in vivo transplantation in non-diabetic animals.
UPLVM and UPLVG capsules, gelled in 50 mM BaCl2 or 120 mM CaCl2, was incubated overnight in islet media (CMRL + 10% newborn calf serum + 5 mM or either BaCl2 or CaCl2) in 37°C 5% CO2 incubator. Capsule size was measured after the overnight incubation, and 14 days after intraperitoneal transplant in either athymic nude (immunodeficient) or CD1 (immunocompetent) mice. All results are shown as mean ± SEM.
Effect of Gelling Ion Type and Concentration after in vivo Transplant of Encapsulated Islets in Diabetic Mice
To determine whether barium or calcium had an effect on the success of transplanted encapsulated islets, barium and calcium gelled encapsulated rat islets in UPLVM alginate was transplanted into immune competent CD1 mice made diabetic using streptozotocin. All recipient mice showed significant reduction in blood glucose, compared to control untreated diabetic mice. Barium-gelled encapsulated islets transplant resulted in significantly lower blood glucose when compared to calcium-gelled encapsulated islets transplant (Figure 3A) At 14 days, there is no significant difference between islet viability in either type of gelling ion transplanted groups at the time of explant. (Figure 3B).
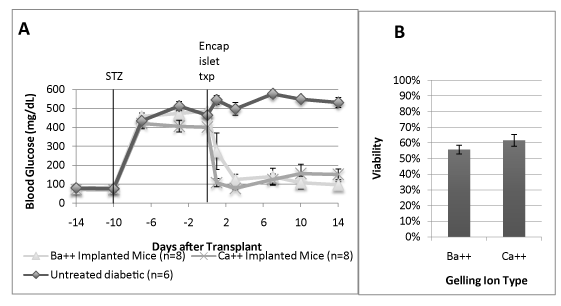
Figure 3. Gelling ion variations and their effect on encapsulated islet efficacy after in vivo transplantation in diabetic animals.
Rat islets were encapsulated in UPLVM alginate, gelled in 50 mM BaCl2 or 120 mM CaCl2, and incubated overnight in islet media (CMRL + 10% newborn calf serum + 5 mM or either BaCl2 or CaCl2) in 37°C 5% CO2 incubator. The encapsulated islets were transplanted in streptozotocin induced diabetic CD1 mice. (A) Non-fasting daily blood glucose level; (B) Viability of explanted encapsulated islets on day 14 measured by fluorescein diacetate/propidium iodide fluorescent staining. All results are shown as mean ± SEM.
High mannurate (UPLVM)2021 Copyright OAT. All rights reserv bath exhibited sodium-dependent isotropic swelling not seen in the barium gelled capsules in both in-vitro trials and in-vivo trials. High glucuronic acid alginate (UPLVG) capsules gelled in both barium and calcium did not exhibit swelling in-vitro, but calcium gelled UPLVG capsules exhibited isotropic swelling in athymic nude mice. The ability to optimize alginate microcapsule morphology is an important step in development clinical-grade encapsulation strategies for the treatment of diabetes. Isotropic swelling in alginate microcapsules has profound implications on the morphology of the microcapsules and thus the efficacy of the islets encapsulated within the microcapsules. Isotropic swelling within the alginate microcapsules will decrease the surface area to the volume ratio of the capsule. A higher surface area to volume ratio is needed in these capsules in order to maximize the transfer of nutrients needed to sustain islet viability. A decrease in the surface area to volume ratio decreases the efficacy of nutrient transport with the islets and thus increases the chance of islet hypoxia. Hypoxia would result in a decrease of insulin secreted [15] as well as increase in apoptosis [16]. As noted, islets encapsulated in barium-gelled alginate showed a significantly lower blood sugar in CD1 mice after 14 days compared to calcium-gelled islets. It is also noted that there is no significant difference between the viability of islets between barium-gelled capsules and calcium-gelled capsules.
The ability to optimize alginate microcapsule morphology is an important step in development of clinical-grade encapsulation strategies for the treatment of diabetes [17, 18]. Isotropic swelling noted at physiological sodium ion concentrations is expected to affect microcapsule permeability, which has profound implications for encapsulated islet survival after transplantation. Assays that can evaluate microcapsule permselectivity could be performed to determine the optimal culture conditions to achieve the ideal pore size to maximize transplant success in encapsulated islets [19-21]. The results of this study suggest that alginate composition (specifically, guluronic acid content) and gelling ion type and concentration greatly influence microcapsule morphology and stability. These results indicate that high mannuronate alginate microcapsules and gelling in Ba++ confer significantly better protection from microcapsule swelling.
The ability to optimize alginate microcapsule morphology is an important step in development of clinical-grade encapsulation strategies for the treatment of diabetes. Since microcapsules are being evaluated for use in transplantation studies, it would be pertinent to develop strategies to mitigate sodium-induced isotropic swelling in alginate microcapsules. Studies are also underway to determine whether sodium-induced isotropic swelling in alginate microcapsules impacts microcapsule stability, permeability and permselectivity. The effects of alginate type and choice of gelling ion directly influence microcapsule size during in vitro incubation in physiological solutions. Since microcapsules are being evaluated for use in transplantation studies, it would be pertinent to develop strategies to further evaluate barium-gelled alginate UPLVM and UPLVG microcapsules with in vivo studies to evaluate their safety and biocompatibility for islet and stem cell transplantation. However, there remain a variety of parameters to be optimized such as the alginate purification processes, composition, use of engraftable poly-amino acids to achieve modifications to microcapsule surface and configuration, identification of the optimal transplantation site, and identification of the best islet donor before successful translation to human clinical trials can be achieved.
The authors gratefully acknowledge funding from the Juvenile Diabetes Research Foundation grant #17-2013-288. The authors also acknowledge support from the Department of Surgery, and the Sue and Bill Gross Stem Cell Center at the University of California, Irvine. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR001414, for support with statistic analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
- National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. (2001). Atlanta, GA: US Department of Health and Human Services. [Crossref]
- Wild S, Roglic G, Green A, Sicree R and King H. (2004). Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047-1053. [crossref]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM and Rajotte RV. (2000). Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 343(4):230-238. [Crossref]
- Manz B, Hillgärtner M, Zimmermann H, Zimmermann D, Volke F and Zimmermann U. (2004). Cross-linking properties of alginate gels determined by using advanced NMR imaging and Cu(2+) as contrast agent. Eur Biophys J 33: 50-58. [crossref]
- Rother KI and Harlan DM. (2004). Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest 114: 877-883. [crossref]
- Scharp DW and Marchetti P. (2014). Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 67-68:35-73. [Crossref]
- Darrabie MD, Kendall WF and Opara EC. (2006). Effect of alginate composition and gelling cation on microbead swelling. J Microencapsul. 23(6):613-621. [Crossref]
- Kendall W, Darrabie M, Freeman B, Hobbs H, Collins B and Opara E. (2000). Effect of bead swelling on the durability of polylysine alginate microcapsules. Curr Surg 57: 636-637. [crossref]
- Zimmermann H, Wahlisch F, Baier C, Westhoff M, Reuss R, Zimmermann D, Behringer M, Ehrhart F, Katsen-Globa A, Giese C, Marx U, Sukhorukov VL, Vasquez JA, Jakob P, Shirley SG and Zimmermann U. (2007). Physical and biological properties of barium cross-linked alginate membranes. Biomaterials. 28(7):1327-1345. [Crossref]
- Rosiński S, Grigorescu G, Lewińska D, Ritzén LG, Viernstein H, Teunou E, Poncelet D, Zhang Z, Fan X, Serp D, Marison I and Hunkeler D. (2002). Characterization of microcapsules: recommended methods based on round-robin testing. J Microencapsul 19: 641-659. [crossref]
- De Vos P, Spasojevic M and Faas MM (2010) Treatment of diabetes with encapsulated islets. Adv Exp Med Biol 670: 38-53. [crossref]
- Krishnan R, Arora RP, Alexander M, White SM, Lamb MW, Foster CE, 3rd, Choi B and Lakey JR. (2014). Noninvasive evaluation of the vascular response to transplantation of alginate encapsulated islets using the dorsal skin-fold model. Biomaterials. 35(3):891-898. [Crossref]
- Weber CJ, Zabinsi S, Koschitzky T, Rajotte R, Wicker L, Peterson L, D'Agati V and Reemtsma K. (1990) Microencapsulated dog and rat islet xenografts into streptozotocin-diabetic and NOD mice. Horm Metab Res Suppl 25: 219-226. [crossref]
- Lamb M, Storrs R, Li S, Liang O, Laugenour K, Dorian R, Chapman D, Ichii H, Imagawa D, Foster C 3rd, King S and Lakey JR. (2011). Function and viability of human islets encapsulated in alginate sheets: In vitro and in vivo culture. Transplant Proc. 43(9):3265-3266.
- Dionne KE, Colton CK and Yarmush ML. (1993). Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42: 12-21. [crossref]
- Ah Kim H, Lee S, Park JH, Lee S, Lee BW, Ihm SH, Kim TI, Kim SW, Ko KS and Lee M. (2009). Enhanced protection of ins-1 ß-cells from apoptosis under hypoxia by delivery of DNA encoding secretion signal peptide-linked exendin-4. J Drug Target. 17(3):242-248. [Crossref]
- Tam SK, Bilodeau S, Dusseault J, Langlois G, Hallé JP and Yahia LH. (2011). Biocompatibility and physicochemical characteristics of alginate-polycation microcapsules. Acta Biomater 7: 1683-1692. [crossref]
- Wang JY, Jin Y, Xie R, Liu JY, Ju XJ, et al. (2011) Novel calcium-alginate capsules with aqueous core and thermo-responsive membrane. J Colloid Interface Sci 353: 61-68. [crossref]
- Cellesi F, Weber W, Fussenegger M, Hubbell JA and Tirelli N. (2004). Towards a fully synthetic substitute of alginate: Optimization of a thermal gelation/chemical cross-linking scheme ("tandem" gelation) for the production of beads and liquid-core capsules. Biotechnol Bioeng. 88(6):740-749. [Crossref]
- Paolo Blasi, Stefano Giovagnoli, Aurélie Schoubben, Maurizio Ricci, Carlo Rossi, Giovanni Luca, Giuseppe Basta and Riccardo Calafiore. (2006). Preparation and in vitro and in vivo characterization of composite microcapsules for cell encapsulation. Int J Pharm 324: 27-36. [crossref]
- Mørch YA, Donati I, Strand BL and Skjåk-Braek G. (2006). Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 7: 1471-1480. [crossref]


