Abstract
The mesoporous Copper, nitrogen co-doped TiO2 microspheres was prepared via solvothermal approach, followed by nitriding treatment under an ammonia gas flow. The crystalline structures of the as-prepared catalyst and the chemical compositions of Cu,N co-doped TiO2 were determined using X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) respectively. The photocatalytic activity of the as-prepared sample was investigated by monitoring the degradation of Rhodamine-B under visible light irradiation.
Experimental results indicated that mesoporous Cu,N co-doped TiO2 microspheres showed higher photocatalytic activity than Cu-TiO2 microspheres and anatase TiO2 under visible light irradiation. The higher photocatalytic activity of the mesoporous Cu,N co-doped TiO2 microspheres sample could be attributed to the synergistic effects of large BET surface area, extended light absorption, efficient charge separation which was stabilized by the presence of oxygen vacancies. It was discovered that, valence states maintain stability after nitriding treatment. The sample synthesized from 0.1% molar quantity of Cu dopant, and nitrided at 400℃ for 30 min gave the highest photocatalytic activity.
Key words
Visible light photo-catalysis, TiO2, nanostructure, nitridation, co-doping, photodegradation
Introduction
Titanium dioxide (TiO2) nanomaterials are the most widely used semiconductor in many applications such as energy storages, sensors, photovoltaics and photo-catalysis [1] because of its unique photo-electric properties, high chemical stability, low cost and environmental friendly [2-6]. However, photo-catalytic performance of TiO2 is relatively low due to fast recombination of electron-hole pairs and narrow light response range [7] compare to noble metal modified TiO2 which has been proved to be very effective in overcoming this drawback [8-10]. Since the lower Fermi level of noble metals resulting in an efficient separation of charge carriers, enhanced performance of photocatalysis could be achieved. However, noble metals are expensive and rare; hence, identification of metals with similar enhancement for photo-catalytic activity increasingly draws research attentions. Cu is much cheaper compared to noble metals, and Cu-TiO2 has been extensively studied for the photodegradation of organic pollutants [11-14]. The electrons generated from the excitation of photocatalyst in the valence band (VB) of TiO2 are directly transferred to Cu2+ under conduction band, which frees up the oxidative valence hole of the photocatalyst for the degradation of organic compounds. Metal ion dopants can serve as charge trapping sites and thus reduce electron-hole recombination rate during photocatalysis [15].
Recent years, many researchers also pay attention to dope TiO2 with non-metals such as S, N, P and B [16-21]. Among all the non-metals-doped TiO2 catalysts, N-doped TiO2 had been intensively investigated. The N-doped pathway could form intermediate energy levels in the band gap as a consequence of either mixing N 2p with O 2p states or by introducing localized states. Comparing with single doping, co-doping of metal(s) and non-metal(s) on TiO2 shows better performances in photocatalysis due to the synergetic effects [22-24] of large BET surface area and extended light absorption.
In our research work, we reported mesoporous Cu,N co-doped TiO2 microspheres that showed relatively high catalytic activity. Here, we present a green solvothermal approach for the synthesis of mesoporous Cu doped TiO2 microspheres. Nitrogen was doped on Cu-TiO2 microspheres by nitriding treatment under an ammonia gas flow. The as-prepared sample could efficiently overcome the drawbacks mentioned above to some extent, because it has high sufarce area, effective charge separation and ability to absorb light at visible light region. Mesoporous structures with high surface area can provide an excellent support for active sites due to pore sizes and volume. Photocatalytic activity of the as-prepared catalyst was investigated by monitoring the degradation of Rhodamine-B (RhB) under visible light irradiation.
Experimental section
Materials and methods
All chemicals were analytical grade reagents and used without further purification. Hexadecylamine (HDA; 90%), Titanium (IV) isopropoxide (TIP; 98%), Copper chloride (CuCl2), absolute ethanol, deionized water, potassium chloride(AR) and anatase TiO2 were supplied by the Sigma-Aldrich, China. Rhodamine-B was used as model organic pollutant to evaluate the photocatalytic activity of the synthesized materials, and double distilled water was used throughout the experiment.
Synthesis of mesoporous Cu-doped TiO2 microspheres (Cu-TiO2): Mesoporous Cu-TiO2 microspheres were synthesized via solvothermal reaction of TIP, HDA, KCl, and CuCl2, followed by calcination. Firstly, mixture of CuCl2 and TIP in molar ratios: 0.01, 0.02, 0.05, 0.1, 0.5, 1, 3, and 10, 1.98 g HDA and 1.60 mL of 1.6 M KCl solution were dispersed in 200 mL ethanol under stirring and the sample was allowed to react for 30 min. TIP (4.5 mL) as Titanium source was slowly dripped into the mixture under stirring. After 2 min, the white precursor bead in suspension was kept static for 18 h, centrifuged and washed with ethanol three times, and the sample was then air-dried at room temperature. After that, the sample was transferred into a Teflon-lined autoclave with a capacity of 100 mL. The autoclave was sealed, transferred to electric muffle furnace and kept at 160°C for 6 h. The precipitate obtained was collected by the centrifugation, washed with ethanol and air-dried. Finally, these powders obtained were calcined at 500℃ for 2 h leading to the formation of Cu-TiO2 microspheres. In this research work, TiO2:(0.1 at% Cu) stands for 0.1 at % Cu loaded TiO2 sample wherein the % is to be interpreted in terms of the molar ratio mentioned earlier in this paragraph.
Synthesis of Mesoporous Cu, N Co-doped TiO2 microspheres (Cu-N-TiO2): The sample obtained TiO2:(0.1 at% Cu) was placed in a quartz boat. The boat was placed in a quartz tube with airtight, stainless steel end-caps that have welded valves and connections to input and output gas lines. The quartz tube was placed in a tube furnace and appropriate connection to the gas sources was made. An argon gas was allowed to flow through the tube for 15 min to expel air in the tube before establishing the flow of ammonia gas through the tube. The sample was then heated in the tube at 400°C, 500°C and 600°C with heating rate 4°C/min. After 30 min or 2 h elapsed, the furnace was turned off and the product was cooled to room temperature within 4 h under ammonia gas flow. Before the quartz tube was taken out of the tube furnace, argon gas was allowed to flow through the tube in order to expel the remaining ammonia gas in the tube. In this research work, TiO2:(0.1 at% Cu,N - T - t) stands for 0.1 at % Cu doped TiO2 with nitriding treatment time t = 30 min or 2 h and calcined at T = 400, 500 and 600°C.
Characterization
The crystalline structures of the sample was examined with X-ray diffraction (XRD) using X-ray Diffraction (XRD) with Miniflex600 X-ray diffractometer containing monochromatic Cu Kα radiation (λ=0.1542 nm, accelerating voltage 40 kV, applied current 15 mA) at scanning rate of 1°/min. The morphology and chemical composition were determined using scanning electron microscopy (SEM) instrument (JSM-7800F, Japan) and X-ray Photoelectron Spectroscopy (XPS) respectively. XPS measurements were carried out on an X-ray photoelectron spectrometer (ESCALAB250Xi) using Al Kα (1486.6 eV) X-rays as the excitation source. Carbon 1s (284.6 eV) was chosen as reference. UV-vis diffuse reflectance spectra were recorded with a Hitachi U-3900 spectrometer in the range of 200-850 nm, using BaSO4 standard as reference. Surface area measurements were carried out by using nitrogen adsorption-desorption technique of Brunauer-Emmet-Teller (BET) method on Accelerated Surface Area and Porosimetry System (ASAP 2420) to obtain value of specific surface area, pore volumes and mean value of pore sizes.
Determination of photocatalytic properties and degradation of Rhodamine-B (RhB)
Photocatalytic properties were determined in an open thermostatic photo-reactor. Before light irradiation, 200 mL of suspension solution containing 10 mg/L RhB and 120 mg solid catalyst was sonicated for 10 min, and then stirred for 1 h in the darkness to ensure an adsorption–desorption equilibrium was maintained. The suspension was then irradiated under continuous stirring using UV light, 300 W Micro-solar 300UV - Xe lamp with a UV-cut off filter and was positioned 25 cm away from the reactor (removing the effect of light on the photocatalysis) in order to allow only visible light in the range of 420 nm-850 nm to interact with the sample, and the experiment was carried out at 25°C under constant stirring. At a given time, interval of irradiation, 5 mL of the solution was withdrawn, centrifuged and analysis with a UV-vis absorption (Hitachi U-3900 spectrometer) at the maximal absorption wavelength for RhB, which have characteristic absorption peaks of 554 nm (λRhB). To establish the stability of the photocatalyst, the mesoporous Cu-N-TiO2 spheres was recycled and used three times for testing subsequent photocatalytic activities as follows; After a photocatalytic experiment, the mesoporous Cu-N-TiO2 microspheres was recovered by washing with distilled water three times and dried at 80°C for 12 h to remove the residual reactants and reactivated the adsorption and catalytic performance.
Results and discussion
Crystal phases and morphology of the Cu-TiO2 microspheres
Crystalline phases of the samples with different molar mass of Cu2+ were investigated with XRD, as shown in Figure S1. All the diffraction peaks are index to anatase TiO2 structure (JCPDS card NO.21-1272) with five main characteristic peaks; the planes (101), (004), (200), (105) and (211) respectively. No impurity was detected except in 10 at% Cu-TiO2 sample, and this indicated that copper dopant has a negligible effect on the crystalline phases of TiO2 microspheres. The diffractograms of all the samples were not shown any diffraction peaks of copper or copper compounds except the one of 10% Cu-TiO2 which could be attributed to low copper content in these samples. In the present study, the Cu is in +2 oxidation state and Ti is in +4 oxidation state. The radius of Ti4+ (0.68 Å) is similar to that of Cu2+ (0.73 Å), hence copper ions may be incorporated into the lattice of TiO2 and occupied some of the titanium lattices [25,26]. The charge compensation is mainly achieved by the ionized vacancies especially doubly ionized oxygen vacancies. As shown in Figure 2, in order to further enhance the photocatalytic activity of TiO2: (0.1 at% Cu) sample in the visible light region, nitriding treatment were carried out under ammonia gas flow. The PXRD patterns of TiO2:(0.1 at% Cu, N-400°C-30 min) sample shows a good crystallinity, and the PXRD patterns of mesoporous TiO2:(0.1 at% Cu, N) microspheres with different nitriding conditions are shown in Figure S2.
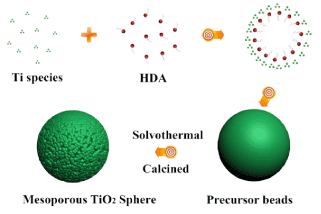
Figure 1. possible mechanism for beads growth.
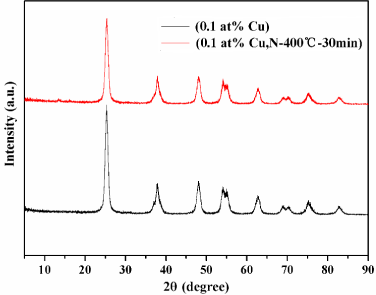
Figure 2. XRD patterns of TiO2:(0.1 at% Cu) and TiO2:(0.1 at% Cu, N-400°C-30 min) microspheres.
All the samples exhibit good crystallinity of anatase TiO2 diffraction peaks (JCPDS no. 21-1272) with no other peaks, demonstrating phase purity, because the temperature and time of nitriding treatment were not sufficient to produce TiN.
Figure 3 shows the scanning electron microscopy (SEM) images (morphology) of the synthesized Cu-TiO2, and Figure 3A, B show the typical FESEM images of the as-synthesized mesoporous TiO2:(0.1 at% Cu) microspheres at different magnifications. During calcinations, monodispersed TiO2:(0.1 at% Cu) microspheres with a diameter of (0.6 ± 0.05) μm, and comparatively rough surfaces are produced (Figure 3A) owing to the removal of the template. As illustrated by the high magnification SEM image (Figure 3B), TiO2 beads contain nanocrystals and pores were obviously observed over the surface of the beads. This special structure is beneficial by allowing light scattering on the surface and in the pores of the beads. Even after nitriding treatment at 500°C for 2 h, agglomeration of TiO2:(0.1 at% Cu, N-500°C-2 h) was not obvious, and some microspheres were broken. However, the beads still retained mesoporous structures as shown in Figure 3C, D.
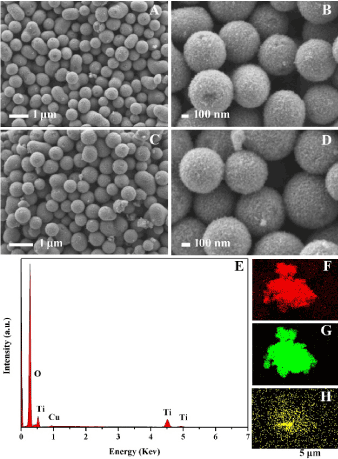
Figure 3. Typical FESEM (A and B) of TiO2:(0.1 at% Cu) and (C and D) of TiO2:(0.1 at% Cu, N-500°C-2 h) samples, EDS mapping (E, F(O), G(Ti) and H(Cu)) images of TiO2:(3 at% Cu) sample.
The EDS spectrum (Figure 3E) indicated that the composite consists of Cu, Ti and O as it was revealed by EDS technique. Moreover, the elemental mapping of TiO2:(3 at% Cu) was also performed by EDS area scanning and amount of Cu present was too low to be detected. So, we choose a relative high Cu content sample (3 at% Cu) in order to estimate amount of copper present in the sample by using EDS. The maps (Figure 3F, G and H) of O, Ti, and Cu are well defined with sharp contrast, and the profile of Cu is close to that of O and Ti, which indicates that Cu and Ti are distributed uniformly and densely throughout the whole composite.
BET surface area and pore size distribution of the Cu-TiO2 microspheres
To examine the porosity of the TiO2:(0.1 at% Cu) and TiO2:(0.1 at% Cu, N-500°C-2 h), N2 adsorption-desorption isotherms were performed, and results obtained are shown in Figure S3. The corresponding mono-modal pore-size distributions (inset, Figure S3) indicates the mesoporous nature of the two samples with the pore sizes smaller than 10 nm, and after nitriding treatment the pores were almost not changed. As a result, the mesoporous TiO2:(0.1 at% Cu) microspheres has a high BET surface area of 113.3 m2g-1 owing to the presence of mesoporous nature. Furthermore, after nitriding treatment under ammonia gas, the as-prepared TiO2:(0.1 at% Cu, N-500°C-2 h) sample was also investigated using BET technique, and it is important to note that surface area of the sample is 109.4 m2g-1 and there was no significant decrease in the value except a slight change after nitriding treatment, which might due to the nitriding temperatures. It was discovered from results that all obtained Cu-TiO2 microspheres have excellent surface and mesoporous properties, which might lead to high photocatalytic activities.
Formation process of Cu-TiO2 microspheres
Figure 1 shows a schematic diagram of a possible mechanism for the beads growth process. Based on these results, we propose that the formation of mesoporous spheres proceeds through keeping static, solvothermal and calcination reaction. The mesostructures and monodisperse precursor beads were formed through a cooperative assembly process involving long-chain alkylamine and Ti(OCH(CH3)2)4-x(OH)x species. The resultant Ti(OCH(CH3)2)4-x(OH)x species on hydrolysis of Titanium (IV) isopropoxide (TIP) participate in hydrogen-bonding interactions with amino groups of the Hexadecylamine (HDA), such hybrid composites contain hydrophobic long-chain of alkyl groups. Meanwhile, further hydrolysis and condensation of the titanium species associated with the hybrid micelles resulted into the formation of a new liquid condensed phase rich in HDA which can be referred to as titanium oligomers. As the titanium oligomers further polymerized, the condensed phase became denser, and this might due to effect of ammonia released during hydrolysis hastening decomposition of titanium oligomers leading to the formation of mesostructured inorganic frameworks which finally precipitated out of the solvent. During solvothermal treatment, amorphous TiO2 was known to experience a phase change to anatase via a dissolution and reprecipitation processes, wherein dissolved titanate species rapidly nucleated to form nanocrystalline structures due to the high solvothermal system. Further calcination in air induces decomposition of HDA molecules and promotes the formation of well crystalline mesoporous TiO2 sphere.
Optical properties
Optical absorption properties: The optical absorption properties of the pristine and Cu doped TiO2 catalysts were investigated by the comparison of the UV-vis diffuse reflectance spectra (DRS) as shown in Figure S4A. And it was observed that increase in Cu content brought-about shifting of absorption edge to visible light region and intense absorption, and this intense visible light absorption can be attributed to: (i) the Cu doping introduced impurity states below the conduction band minimum leading to the band gap reduction. (ii) the excitations between O 2p states and Ti 3d states through Cu 3d states. As TiO2+ is an indirect semiconductor, the band-gap energies of as-prepared Cu-doped TiO2 samples estimated from the Tauc plot of [F(R∞)*hν]1/2 versus energy (E) was shown in Figure S4B. After doping of Cu2+ ions into TiO2 matrix, the sample changed from white (TiO2) to light gray. The Eg value of Cu-TiO2 samples decrease as Cu contents increase as shown in FigureS4B, and this indicates that the samples show visible-light response.
Photodegradation of Rhodamine-B: Rhodamin-B (RhB) is one of the most commonly used organic dyes in industry and can pollute the environment. The photocatalytic activity of the Cu doped TiO2 samples was initially evaluated by the degradation of RhB in an aqueous solution (Figure 4A). In addition, prepared mesoporous TiO2 microspheres was used as a reference and the adsorption–desorption result of the catalyst without light irradiation showed that there was almost no change in the pollutant concentration after 60 min which indicated that an adsorption–desorption equilibrium was reached. Also, the photocatalytic degradation efficiency (PDE = (C0-Ct)/C0) of RhB hardly changed under visible light irradiation in the absence of the photocatalyst. Use of as-prepared samples for degradation of RhB shows that the photocatalytic activity of all Cu-TiO2 samples outperformed TiO2 sample, and TiO2:(0.1 at% Cu) sample was the best one of them. Figure 4B shows representative variations in the absorption of RhB (λmax = 554 nm) under visible light irradiation for the TiO2:(0.1 at% Cu) sample as catalyst, the characteristic absorption of RhB gradually decreases as the irradiation time increases, and the characteristic wavelength shifts to a lower wavelength (inset, Figure 4B) which indicates that RhB decomposed and new substance produced.
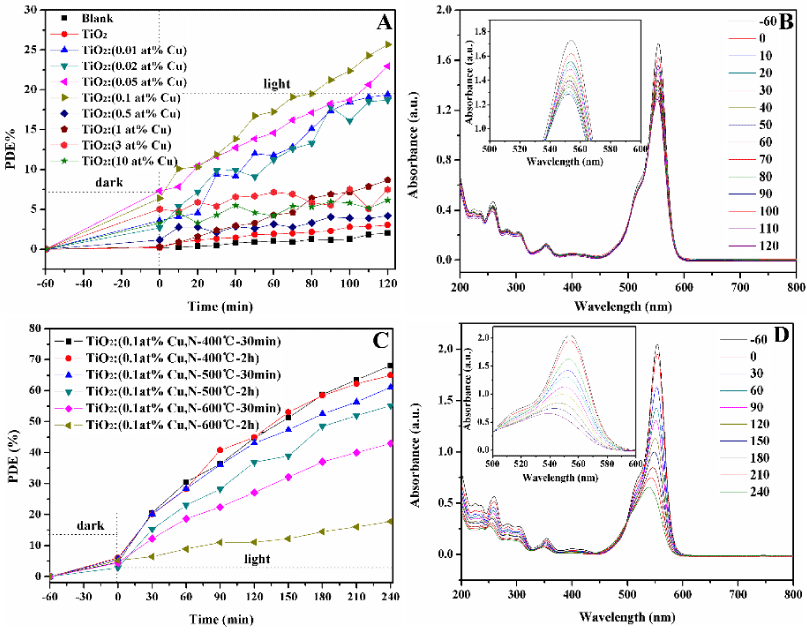
Figure 4. Time-dependent photocatalytic degradation of RhB (A) solution upon exposure to visible light using the obtained Cu-TiO2 samples, Representative variations of the characteristic absorption of RhB (B) under visible light irradiation by using TiO2:(0.1 at% Cu) sample (-60 to 0 as absorb time, 0-120 as photocatalysis time). Time-dependent photocatalytic degradation of RhB (C) solution upon exposure to visible light using the obtained Cu-N-TiO2 samples, Representative variations of the characteristic absorption of RhB (D) under visible light irradiation by using TiO2:(0.1 at% Cu, N-400°C-30 min) (-60 to 0 as absorb time, 0-240 as photocatalysis time).
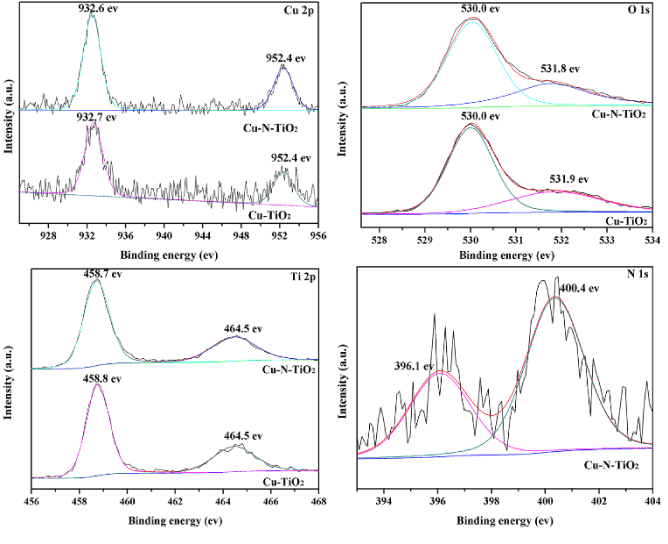
Figure 5. XPS spectra for (A) Cu (2p), (B) O(1s), (C) Ti(2p) and (D) N(1s) signals of TiO2:(0.5 at% Cu, N-400°C-30min).
In order to investigate the photocatalytic activity of as-prepared samples thoroughly, a kinetic study was performed for the photodegradation process. This is fitted by using pseudo-first-order kinetics, and the linear relationship of ln(C/C0) vs time is used to calculate apparent rate constants. The results from these fitting exercises are displayed in Table 1, and the results imply that k-values 1.82 × 10-3 min-1 for TiO2:(0.1 at% Cu) sample is higher than those of the other Cu-TiO2 samples. And this is in agreement with results that show that TiO2:(0.1 at% Cu) sample has best photocatalytic activity.
Table 1. The k-values of the as-prepared TiO2 and Cu-TiO2 samples.
Catalyst |
kRhB(10-3 min-1) |
TiO2 |
-- |
TiO2:(0.01 at% Cu) |
1.64 |
TiO2:(0.02 at% Cu) |
1.44 |
TiO2:(0.05 at% Cu) |
1.44 |
TiO2:(0.1 at% Cu) |
1.82 |
TiO2:(0.5 at% Cu) |
0.20 |
TiO2:(1 at% Cu) |
0.74 |
TiO2:(3 at% Cu) |
0.13 |
TiO2:(10 at% Cu) |
0.20 |
Based on the results of photocatalytic activity, the mechanism of the photocatalysis of Cu-TiO2 samples is discussed as follow: The dopant Cu generates an impurity level of Cu2+ below the conduction band of TiO2, and the electron in the valence band may be excited to the Cu2+ trap level with the same amount of positively charge holes left to form electron-hole pairs during light irradiation. As a consequence of this proximity, the trapped electron in Cu2+ could easily be released and transferred to a neighboring surficial Ti4+, and the effective charge transfer might decrease the electron-hole pair recombination rate and prolonged the lifetime of charge carriers. In addition, to maintain charge neutrality, oxygen vacancies were generated by doping of Cu2+ in the lattice of TiO2 and oxygen vacancies induced visible light absorption. The recombination rate of the trapped charge carriers’ increases with the dopant concentration, because the average distance between the trapping sites of the two types decreases with increasing in number of dopants confined within a particle. Besides, there might be risk of reducing number of photoactive sites in the sample with too much Cu.
In addition, the mesoporous structure of the as-prepared samples results in multiple reflections of visible light within the hole, allowing the light source to be used more efficiently, which has been reported by many researchers [27]. The high BET surface area 113.3 m2g-1 of TiO2:(0.1 at% Cu) sample may offer enough photoactive sites and promote the reaction.
In order to enhance and increase the photo reactivity of TiO2:(0.1 at% Cu) sample by extending the visible light absorption, nitriding treatment was carried out under ammonia gas, and TiO2:(0.1 at % Cu, N-400oC-30 min) showed best photocatalytic activity, but the copper content was too low to be determined using XPS. Therefore, TiO2:(0.5 at %Cu, N-400oC-30 min) was used to examine the copper content in the sample, and the XPS analysis was also carried out to confirm that the N doped onto mesoporous TiO2:(0.5 at% Cu) microsphere. Meanwhile, the N peak is not obvious in EDS due to the low content of N in TiO2:(0.1 at% Cu, N-400°C-2 h) sample. Figure 5 shows the XPS of Ti 2p, O 1s, N 1s and Cu 2p for the as-synthesized mesoporous TiO2:(0.5 at% Cu, N-500°C-2 h) microspheres. In Figure 5A, the XPS spectrum shows complex structure 932.7 eV for Cu 2p3/2, and 952.4 eV for Cu 2p1/2 of TiO2:( 0.5 at% Cu), and this shows that Cu was incorporated into the lattice of TiO2. Before or after nitriding treatment the peaks of Cu 2p was almost not changed, and Figure 5B shows O 1s peaks at 530.0 and 531.8 eV, the peak at 530.0 eV was assigned to the Ti-O bonds in the TiO2 lattice and the peak at 531.8 eV was related to the hydroxyl groups or water adsorbed on TiO2 surfaces. After nitriding treatment, the peaks of O 1s have little changes from 531.8 eV to 531.9 eV. Figure 5C shows Ti 2p spectrum, in which two peaks are observed at 458.7 and 464.4 eV for Cu-TiO2, which correspond to the binding energies of Ti 2p3/2 and Ti 2p1/2 levels for Ti4+, and Ti3+ spectrum was not observed using XPS. Figure 5D is the N 1s core level spectra of co-doped TiO2, and it was found that there were two peaks with different intensity at the bonding energies of 396.1 eV and 400.4 eV. Generally, the peak at 396.1 eV reflects the formation of N-Ti-O bonds, which indicates the substitution of N-ion for O-ion, and the XPS peak at 400.4 eV could be assigned to interstitial N in TiO2 [28]. In line with the XPS results, the total amount of doped N was 0.75% and substitutional N was 0.27%. It was difficult to make the substitution of O for N because the ionic radius of N(1.71 Å) was much bigger compared to that of O(1.4 Å) [29]. As it was reported by our previous work, the substitutional N and interstitial N play a key role in the band gap narrowing and contributed to the visible light photocatalytic degradation of RhB.
In Figure S5A, the absorption spectrum of TiO2:(0.1 at% Cu, N-400°C-30 min) and TiO2:(0.1 at% Cu,N-400°C-2 h) samples were nearly identical, and the absorption edge in the wavelength increased to 480 nm and 500 nm as the NH3 treatment temperature and duration increased to 500°C for 30 min and 2 h. And an add-on shoulder was imposed onto the edge of the absorption spectrum. With an increase in the nitriding temperature and duration, the color of TiO2:(0.1 at% Cu, N-600°C-2 h) sample showed a dark green color and a higher absorption intensity produced. As shown in Figure S5B, the band gap of TiO2:(0.1 at% Cu, N-400°C-30 min) was almost not changed compared to the one before nitriding treatment, and the Eg value of TiO2:(0.1 at% Cu, N) samples after nitriding treatment gradually decreased with increasing in temperature and duration. As indicated by XPS analysis, there are two kinds of N doping, which are substitutional doping and interstitial doping. For the substitutional doping, N 2p states mixed with O 2p states in the valence band and improved the photoreactivity through narrowing of the inherent band gap of TiO2 to maintain the electroneutrality. The oxygen vacancies give rise to the states below the conduction edge while the interstitial nitrogen atoms induced the local states near the conduction edge. After co-doping with Cu and N, electrons could be excited from valence band to the doping Cu2+ energy level and from the N 2p energy level to the conduction band. Besides, it is possible that the electrons can migrate from the N 2p energy level to the doping Cu2+ energy level, and as a result more photoinduced charge carriers could be effectively separated to participate in the photocatalytic process, leading to a higher photocatalytic activity than Cu-TiO2 samples.
The photocatalytic activities of the TiO2:(0.1 at% Cu,N) samples after nitriding treatment were evaluated by monitoring the degradation of RhB, and Figure 4C shows the degradation curve of RhB catalyzed by TiO2:(0.1 at% Cu,N) samples. TiO2:(0.1 at% Cu, N-400°C-30 min) sample shows a relatively highest photocatalytic activity, and the degradation efficiency reached 68% after 240 min under visible-light irradiation. Figure 4D shows representative variations in the absorption of RhB at maximum wavelength 554 nm under visible light irradiation of TiO2:( 0.1 at% Cu, N-400°C-30 min) sample. The characteristic absorption of RhB decreases obviously as the irradiation time increases, and the hypochromic shift of the maximum absorption was not obvious, indicating that a dominant cleavage of the whole conjugated chromophore structures produced instead of the N-diethylation and prolonged irradiation time might lead to the complete decomposition of RhB. Also, Figure 4C gives degradation information of RhB by TiO2:(0.1 at% Cu, N) sample with lower values than TiO2:( 0.1 at% Cu, N-400°C-30 min) sample. At low nitriding duration and temperature, the N doped on TiO2 mainly exists in interstitial form, and the local states near the conduction edge induce by the interstitial N (dopant) can capture electron, but this electron can be easily returned which make it impossible to narrow the band gap of TiO2 and barely involved in the photocatalysis. Although, TiO2:(0.1 at% Cu, N-600°C-2 h) sample absorb more visible light, but the higher density of oxygen vacancies as recombination centers lead to the decrease in the photocatalytic activity. A kinetic study was performed for the photodegradation process, and it was fitted by using pseudo-first-order kinetics, in which the value of rate constant k is equal to the corresponding slope of the fitting line. The linear relationship of ln(C/C0) vs. time, and the results of TiO2:(0.1 at% Cu, N) samples after nitriding treatment were displayed in Table 2. The results also imply that k-value for TiO2:(0.1 at% Cu, N-400°C-30 min) sample is higher than that of TiO2:(0.1 at% Cu, N) samples, which is in agreement with the result of photocatalytic degradation.
Table 2. The k-values of the as-prepared Cu-N-TiO2 samples.
Catalyst |
kRhB(10-3min-) |
TiO2:(0.1 at% Cu,N - 400°C - 30 min) |
4.46 |
TiO2:(0.1 at% Cu,N - 400°C - 2h) |
4.18 |
TiO2:(0.1 at% Cu,N - 500°C - 30 min) |
3.54 |
TiO2:(0.1 at% Cu,N - 500°C – 2h) |
3.2 |
TiO2:(0.1 at% Cu,N - 600°C – 30min) |
2.14 |
TiO2:(0.1 at% Cu,N - 600°C - 2h) |
0.56 |
Conclusion
In this work, mesoporous Cu, N codoped TiO2 photocatalysts were prepared via a solvothermal method, followed by calcination at 500°C for 2 h and nitriding treatment under ammonia gas flow. The as-prepared sample has diameter 0.60 ± 0.05 μm with relatively rough surfaces, the BET surface area was 113.3 m2g-1 and its main pore sizes is smaller than 10 nm. It was found that as-prepared mesoporous Cu, N codoped TiO2 microspheres samples showed enhanced photocatalytic activity than pure TiO2 under visible-light irradiation, and the higher photocatalytic activity of the mesoporous Cu, N codoped TiO2 microspheres sample could be attributed to the synergistic effects of the large BET surface area, extended light absorption, efficient charge separation, which stabilized by the presence of oxygen vacancies. From these results, we confirmed that mesoporous Cu, N codoped TiO2 microspheres could be used to promote photodegradation of Rhodamine-B under visible light irradiation.
Acknowledgements
This work was supported by National Natural Science Foundation of China through grant 21471147 and Liaoning Provincial Natural Science Foundation through grant 2014020087. M. Yang would like to thank the National "Thousand Youth Talents" program of China.
References
- Xin G, Pan H, Chen D, Zhang Z, Wen B (2013) Synthesis and photocatalytic activity of N-doped TiO2 produced in a solid phase reaction. J Phys Chem Solids 74: 286-290.
- Salvador A, Pascual-Martí MC, Adell JR, Requeni A, March JG (2000) Analytical methodologies for atomic spectrometric determination of metallic oxides in UV sunscreen creams. J Pharm Biomed Anal 22: 301-306. [Crossref]
- Moon GD, Joo JB, Dahl M, Jung H, Yin Y (2014) Nitridation and layered assembly of hollow tio2 shells for electrochemical energy storage. Adv Funct Mater 24: 848-856.
- Zuo F, Wang L, Wu T, Zhang Z, Borchardt D, et al. (2010) Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J Am Chem Soc 132: 11856-11857. [Crossref]
- Bao SJ, Li CM, Zang JF, Cui XQ, Qiao Y, Guo J, et al. (2008) New nanostructured TiO2 for direct electrochemistry and glucose sensor applications. Adv Funct Mater 18: 591-599.
- Hara K, Dan-oh Y, Kasada C, Ohga Y, Shinpo A, et al. (2004) Effect of additives on the photovoltaic performance of coumarin-dye-sensitized nanocrystalline TiO2 solar cells. Langmuir 20: 4205-10. [Crossref]
- Khalid NR, Ahmed E, Hong Z, Ahmad M, Zhang Y, et al. (2013) Cu-doped TiO2 nanoparticles/graphene composites for efficient visible-light photocatalysis. Ceramics International 39: 7107-13.
- Chiarello GL, Aguirre MH, Selli E (2010) Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J Catal 273: 182-90.
- Guo H, Kemell M, Heikkilä M, Leskelä M (2010) Noble metal-modified TiO2 thin film photocatalyst on porous steel fiber support. Appl Catal B: Environ 95: 358-364.
- Tian B, Shao Z, Ma Y, Zhang J, Chen F (2011) Improving the visible light photocatalytic activity of mesoporous TiO2 via the synergetic effects of B doping and Ag loading. J Phys Chem Solids 72: 1290-1295.
- Choi HJ, Kang M (2007) Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded. Int J Hydrogen Energy 32: 3841-3848.
- Behnajady MA, Eskandarloo H (2013) Characterization and photocatalytic activity of Ag-Cu/TiO2 nanoparticles prepared by sol-gel method. J Nanosci Nanotechnol 13: 548-553. [Crossref]
- Liu Z, Wang Y, Peng X, Li Y, Liu Z, et al. (2012) Photoinduced superhydrophilicity of TiO2 thin film with hierarchical Cu doping. Sci Technol Adv Mater 13: 025001. [Crossref]
- Su XQ, Li J, Zhang ZQ, Yu MM, Yuan L (2015) Cu(II) porphyrins modified TiO2 photocatalysts: Accumulated patterns of Cu(II) porphyrin molecules on the surface of TiO2 and influence on photocatalytic activity. J Alloy Compds 626: 252-259.
- Sreethawong T, Yoshikawa S (2005) Comparative investigation on photocatalytic hydrogen evolution over Cu-, Pd-, and Au-loaded mesoporous TiO2 photocatalysts. Catal Commun 6: 661-668.
- Ohno T, Mitsui T, Matsumura M (2003) Photocatalytic Activity of S-doped TiO2 Photocatalyst under Visible Light. Chem Lett 32: 364-365.
- Wang J, Tafen de N, Lewis JP, Hong Z, Manivannan A, et al. (2009) Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J Am Chem Soc 131: 12290-12297. [Crossref]
- Sun S, Gao P, Yang Y, Yang P, Chen Y, et al. (2016) N-Doped TiO2 Nanobelts with Coexposed (001) and (101) Facets and Their Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. ACS Appl Mater Interfaces 8: 18126-18131. [Crossref]
- Sotelo-Vazquez C, Noor N, Kafizas A, Quesada-Cabrera R, Scanlon DO, et al. (2015) Multifunctional P-Doped TiO2 Films: A New Approach to Self-Cleaning, Transparent Conducting Oxide Materials. Chem Mater 27: 3234-3242.
- Feng H, Zhang MH, Yu LE (2013) Phosphorus-doped TiO2 catalysts with stable anatase-brookite biphase structure: synthesis and photocatalytic performance. J Nanosci Nanotechnol 13: 4981-4989. [Crossref]
- Lu N, Quan X, Li J, Chen S, Yu H, et al. (2007) Fabrication of Boron-Doped TiO2 Nanotube Array Electrode and Investigation of Its Photoelectrochemical Capability. J Phys Chem C 111: 11836-11842.
- Choi H, Shin D, Yeo BC, Song T, Han SS, et al. (2016) Simultaneously Controllable Doping Sites and the Activity of a W–N Codoped TiO2 Photocatalyst. ACS Catal. 6: 2745-2753.
- Jaiswal R, Bharambe J, Patel N, Dashora A, Kothari DC, et al. (2015) Copper and Nitrogen co-doped TiO2 photocatalyst with enhanced optical absorption and catalytic activity. Appl Catal B: Environ. 168-169: 333-341.
- Song K, Zhou J, Bao J, Feng Y (2008) Photocatalytic activity of (copper, nitrogen)‐codoped titanium dioxide nanoparticles. J Am Ceram Soc 91: 1369-1371.
- Kong H, Huang W, Lin H, Lu H, Zhang W (2012) Effect of SnO2‐Sb2O5 Interlayer on Electrochemical Performances of a Ti‐Substrate Lead Dioxide Electrode. Chin J Chem 30: 2059-2065.
- Sathishkumar K, Shanmugam N, Kannadasan N, Cholan S, Viruthagiri G (2015) Synthesis and characterization of Cu2+ doped NiO electrode for supercapacitor application. J Sol-Gel Sci Techn 74: 621-630.
- Yeh SW, Ko HH, Chiang HM, Chen YL, Lee JH, et al. (2014) Characteristics and properties of a novel in situ method of synthesizing mesoporous TiO2 nanopowders by a simple coprecipitation process without adding surfactant. J Alloy Compds 613: 107-116.
- Chong MN, Jin B, Chow CW, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44: 2997-3027. [Crossref]
- Daghrir R, Drogui P, Robert D (2013) Modified TiO2 for environmental photocatalytic applications: a review. Ind Eng Chem Res 52: 3581-3599.





