Small proportion of malaria infected individuals develops severe clinical phenotypes while other doesn't. Cerebral malaria (CM) is a selective phenotype of severe malaria that may clusters in certain families. The main purpose of this study was to localize the gene(s) that control(s) the susceptibility to cerebral malaria in a large inbred Sudanese family with sex members having CM. A total of 58 microsatellite markers were typed in the family. Linkage analysis was done by two-point linkage analysis based on a recessive model under 50% of penetrance. There was no markers with a lod score ≥ 3.0 which is the criterion for significant linkage in families. However, one marker (D3S1580) on chromosome 3 with maximum lod scores of 1.40 at recombination fraction (θ at 0.00). This may indicated that a suggestive or inexclusive linkage of cerebral malaria to chromosome 3q27-29 region.
Cerebral malaria, genetic predisposition, gene mapping, linkage analysis
Cerebral malaria (CM) is a complex disease which results from interaction involving both host and environmental factors, including parasite factors. Early studies of malaria therapy for neuro-syphilis suggested that there was significant inter-individuals variation even in the absence of any previous exposure [1]. Ethnic differences in malaria susceptibility supported a role for host genetics although, The Susceptibility/resistance genes and alleles vary markedly from population to another so that major resistance alleles in one region are often absent from another. Further, genetics of the parasite vary from one region to another. Whereas twin and adoptee studies provided strong evidence that host genes influenced other diseases [2]. Susceptibility/resistance for malaria is highly polygenic were host genes were shown to control blood infection levels and regulate anti-malarial immune responses thus, influence the outcome of malaria infection [3,4].
There is a strong evidence for association between both HLA class I and II alleles and resistance and susceptibility to P. falciparum malaria. The higher frequencies of HLA-B35 in Sardinia suggests it’s protective against malaria [5]. Other studies in West Africa indicated that the HLA-B53 and the HLA class II haplotype DRB1*1302-DQB1*0501 both protect against severe malaria but, do not appear to protect against mild forms of the disease [6]. Furthermore, their frequencies are significantly higher in West African population than in Caucasian or Asian populations [6].
Segregation analyses have shown that blood parasitaemia density is under complex genetic control with evidence of a major gene [3,7]. In two independent studies (sib-pair linkage analysis) blood infection levels were linked to the 5q31-33 chromosomal region. Several studies confirmed the importance of chromosome 5q31-q33 in the genetic control of Plasmodium falciparum blood intensity (PFBI) levels [8,9].
Considering malaria phenotypes, there still no satisfactory explanation for the fact that small proportion of individuals infected with plasmodium falciparum develops CM while other are remain asymptomatic or characterized only by mild symptoms. Many host genes are involved in the control of the internal environment which the parasite faces and the major disadvantage of being parasitized should favor the survival of those host alleles that confer some protection against the parasite. Considerably, more evidences are available to indicate the important of host factors in determining the clinical presentations of malaria infection [10]. The full knowledge of these factors is important not only to understand how the disease evolves within an individual, but is also of great important to understand, from an epidemiological point of view, how it behaves in a given population.
Study area
The study was carried out in three hospitals located in cities of Wad Medani, Sinnar and Singa in central Sudan. Most of severe malarial cases in this area are admitted into one of these three hospitals. 30% of all admitted patients are due to severe malaria [10]. These cities lie along the Blue Nile; Wad medani lies about 184 kilometers south to Khartoum, the capital of Sudan, within the largest irrigated Gezira Agricultural Scheme, Sinnar lies immediately down the Sinnar dam which irrigates Gezira Agricultural Scheme while Singa lies about 120 kilometers down Damazein dam and is bypassed with many canals originated from the dam (Figure 2). Thus, the whole area is a continuous breeding focus for the malaria vector, Anopheles mosquito. This area is endemic for P.falciparum with high seasonal incidence during the rainy season from August to November. A second peak of transmission follows during January to March. Thus the data was collected during the rainy seasons and the second peaks of transmission.
Study subjects
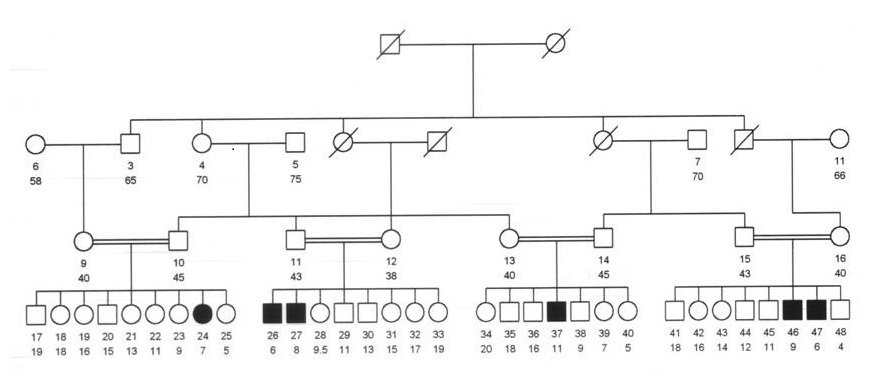
The study subjects belong to 12 different tribal stocks, more than 67% of them belong to only four tribal stocks namely; the Johayna tribal stock (22.8%), Nigerian stock (17.3%), Gaalian stock (16.7%), Nuba stock (11%) (Figure 3). 123 (30%) were offsprings of consanguineous families, either first degree cousins, second degree cousins or far relationship. 100 (24.44%) were clustered in only 42 families which were clustered in only three tribal stocks. 60 (60%) were offsprings of 25 consanguineous families and 6 (6%) were clustering in only one large inbred family (Figure 1).
Ethical Clearance
Ethical approval was obtained both from the Federal Ministry of Health in Khartoum and the University of Gezira Research Committees. Consent was taken from the guardians or parents of all suspected cerebral malaria children admitted to the participating hospitals. Families who gave their consents to participate in the study were followed up in frequent visits. Any participant in the study was free to leave the study at any time.
Definition of Cerebral Malaria (CM) as a clinical phenotype:
Cerebral malaria (CM) is defined as unrousable coma in a child that persisted for more than 30 minutes after any convulsions in the presence of asexual stages of P.falciparum parasite found on thick blood film for which no etiological explanation other than malaria can be attributed. This definition excludes patients suffering from post-ictal coma lasting for less than half an hour after a convulsion, as well as patients suffering from hypoglycemia who had regained consciousness immediately after receiving intravenous glucose. Also, it excludes patients with malaria parasitaemia when coma can be attributed to a concurrent disease such as meningitis, encephalitis, intoxication, etc [11].
Genome scan and Linkage analysis
The main purpose of this study was to localize the gene(s) that control(s) the susceptibility to cerebral malaria in a large inbred Sudanese family with sex members having CM. Family studies have proved to be the most appropriate type of studies to detect genes conferring susceptibility to disease. We selected a large consanguineous family with 6 affected children for linkage analysis (Figure 1). Using ABI PRISM 310 Genotyper, a total of 58 DNA microsatellite markers selected from ABI PRISM®Linkage Mapping Sets were typed in the large inbred family.
Figure 1. The Large inbred family with six affected offspring’s.
For linkage analysis, Two-point analysis was using FASTLINKE package that uses MLINKE program [12]. The pedigree and phenotypic data were processed by a series of preparatory programs in the analytic LINKAGE package.
Linkage analysis for the inbred family
In this study a total of 58 microsatellite markers were typed in the big inbred consanguineous family (Total members of 37). Family 13 was excluded from the analysis by the incompatibility of the mother. Linkage analysis was done by two-point linkage analysis based on a recessive model under 50% of penetrance. There was no markers with a lod score ≥ 3.0 which is the criterion for significant linkage in families proposed by Lander and Kruglyak [13].
Two-point lod scores of CM of markers in chromosome 3
Figure 2
Figure 2. Co-segregation of the genetic markers and CM in chromosome 3 in family.
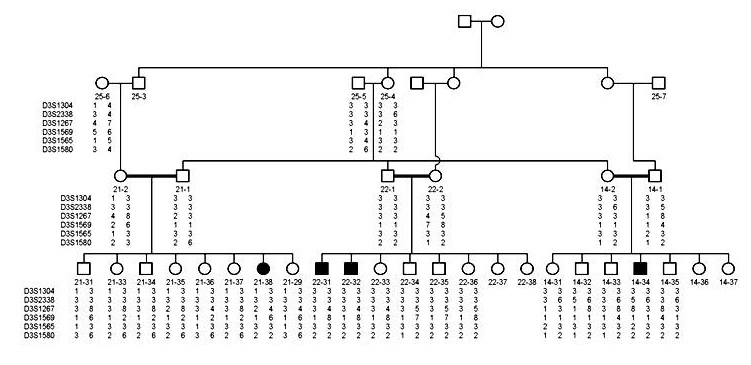
shows the co-segregation of markers and CM phenotype in chromosome 3 in the family. The maximum lod score and recombination fraction for markers typed on chromosome 3 were shown in (Table 1). However, there was one marker (D3S1580) with maximum lod scores of 1.40 at recombination fraction (θ at 0.00). Figure 3 shows the locations of the markers in chromosome 3 and region 3q27-29 where the D3S1580 marker, that gave the lod score of 1.40, is localized.
Figure 3. Co-segregation of the genetic markers and CM in chromosome 3 in family.
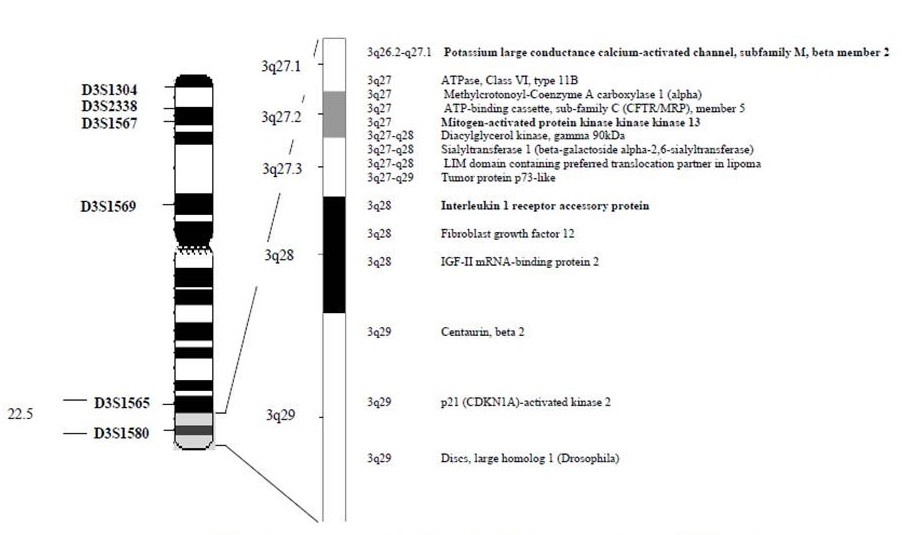
Table 1. Two-point Lod score for chromosome 3 markers.
Lod score at θ |
Marker |
0.0 |
0.01 |
0.05 |
0.1 |
0.2 |
0.3 |
0.4 |
D03S1304 |
-3.21 |
-1.47 |
-0.80 |
-0.53 |
-0.28 |
-0.15 |
-0.06 |
D03S2338 |
-1.96 |
-0.22 |
0.34 |
0.47 |
0.40 |
0.21 |
0.04 |
D03S1267 |
-8.13 |
-3.22 |
-0.85 |
0.10 |
0.75 |
0.80 |
0.53 |
D03S1569 |
-4.40 |
-2.69 |
-1.33 |
-0.63 |
-0.11 |
0.03 |
0.01 |
D03S1565 |
-2.86 |
-1.09 |
-0.47 |
-0.25 |
-0.08 |
-0.02 |
0.00 |
D03S1580 |
1.40 |
1.37 |
1.26 |
1.11 |
0.82 |
0.53 |
0.26 |
Two-point lod scores of CM of markers in 5q31-33 region
The lod scores for the different theta values obtained for the seven markers that were genotyped for 5q31-33 region (Table 2) clearly indicate that there is no linkage between this region and CM phenotype in this inbred family.
Table 2. Two-point Lod score for chromosome 5q31-33 markers.
Lod score at θ |
Marker |
0 |
0.01 |
0.05 |
0.1 |
0.2 |
0.3 |
0.4 |
D5S0471 |
-9.11 |
-2.02 |
-0.16 |
0.44 |
0.69 |
0.56 |
0.29 |
D5S2057 |
-5.41 |
-3.77 |
-1.35 |
-0.42 |
0.21 |
0.3 |
0.19 |
D5S2115 |
-6 |
-4.02 |
-1.65 |
-0.77 |
-0.22 |
-0.11 |
-0.08 |
D5S0436 |
-9.7 |
-4.94 |
-2.32 |
-1.3 |
-0.48 |
-0.18 |
-0.06 |
D5S0636 |
-10.8 |
-3.94 |
-1.97 |
-1.19 |
-0.52 |
-0.21 |
-0.04 |
D5S0410 |
-9.5 |
-3.51 |
-1.59 |
-0.88 |
-0.33 |
-0.13 |
-0.05 |
D5S0422 |
-15.02 |
-5.5 |
-2.82 |
-1.74 |
-0.78 |
-0.33 |
-0.09 |
Suggestive linkage of Cerebral Malaria to 3q27-q29 region
There was one marker (D3S1580) with maximum lod scores of 1.40 at recombination fraction (θ at 0.00) which is the criterion for suggestive or inexclusive linkage of complex inheritance (Table 2) [13-15]. D3S1580 marker is located in 3q27-q29 region which contains several orthologous genes (Figure 3). Two of them, are encoding molecules that play important role in signal transduction and regulation of many cellular processes and gene expression and that may be related to the pathophysiology of CM.
Figure 3. Region 3q27-29 showing the location of D3S1580 markers that gave the lod score (1.40 at θ = 0.0) and the orthologous genes in the region.

Interleukin-1 receptor accessory protein (IL1RAP) is the first candidate gene
Interleukin 1 (IL-1) is related to the immunology of experimental malaria. Animal model studies indicated that IL-1 protects P.berghei-infected mice from lethal CM [16]. Very low concentrations of IL-1, with negligible effects on it’s own, greatly enhanced the effectiveness of TNF-α in particular, in generating nitric oxide, a mediator argued to induce cerebral malaria [17,18]. IL-1 RA improved the outcome in some experimental diseases (endotoxemic shock, cerebral malaria, arthritis, graft-versus-host reaction) [19].
Interleukin-1 receptor accessory protein (IL1RAP) is a transmembrane protein that encoded in the chromosomal region 3q27-29 is a possible candidate gene that controls the susceptibility to CM. It is required for IL-1 signal transduction. The IL-1 system is composed of IL-1 alpha and IL-1 beta, two forms of precursors, two distinct receptors, two soluble fragments of the extramembranous regions of these receptors, and a natural antagonist of IL-1 receptors (IL-1 ra) which could be secreted or remain intracellular. In vitro treatment of cells with IL1 induced the formation of a complex containing IL1RAP and type I IL1 receptor (IL1RA). Interleukin-1 receptor-associated kinase (IRAK) recruits to this complex through its association with IL1RAP and overexpression of mutant IL1RAP proteins lacking the intracellular IRAK-binding domain prevented the recruitment of IRAK to the receptor complex [20].
Potassium channel, Calcium-activated large conductance subfamily, beta member 2 (KCNMB2) is the second candidate gene
Potassium channel, Calcium-activated large conductance subfamily, beta member 2 (KCNMB2). Calcium-activated large conductance subfamily have several roles in cellular metabolism and excitability [21]. Potassium currents play a critical role in action potential repolarization, setting of the resting membrane potential, control of neuronal firing rates, and regulation of neurotransmitter release [22]. Coexpression of KCNMA1 with KCNMB2 induced fast inactivating currents that could be increased by voltage and intracellular calcium and this coexpression lowered the sensitivity of KCNMA1 to change in the affinity of the channel for Ca(2+) [23-25]. Increase in global Ca(2+) in small cerebral blood vessel may leading to increased arterial tone in experimental animal [26]. The involvement of KCNMB2 in many ways in electrolyes balance, indue release of neurotransmitters and arterial tone as mentioned indicated that KCNMB2 may be involved in the control of susceptibility to CM. The KCNMB2 gene was mapped to 3q26.2-q27, in close proximity to the KCNMB3 gene [25]. The KCNMB2 weakly expressed in smooth muscle tissues and in heart, brain, and fetal lung.
These findings pointed out that; this region may play, even a minor, effect in development of cerebral malaria and further studies in this region by typing of additional markers in this big inbred family or more other families will be needed to investigate the linkage of this region with CM.
Absence of linkage between Cerebral Malaria and 5q31-33 region
Lod scores for the different theta values obtained for the seven markers that were genotyped for 5q31-33 region as shown in (Table 3 15). This indicated that no linkage was found between CM and the chromosomal region 5q31-33 in the family. contains chromosomal region 5q31-33 numerous genes encoding immunological molecules such as cytokines, growth factors, and growth-factor receptors which are involved in the control of immunity to P. falciparum blood stages [9,8,27,28]. This region was reported to be linked with control of P. falciparum parasitaemia [6-8,26]. Moreover, the importance of this region in immune regulation is highlighted by its linkage to plasma immunoglobulin E (IgE) levels [30,31]. Comparison of the levels of total IgE or IgE anti-malaria antibodies in patients with uncomplicated malaria with those in patients with the severe form of the disease [32] indicate that these levels are significantly higher in the cases with severe disease [33].
The absence of linkage of this region with CM indicates that anti-disease immunity and anti-infection immunity may be under control by distinct and different genetic factors. In addition P. falciparum parasitaemia is not always correlated with the severity of the disease [34]. Furthermore, population differences in both the host and the parasite may contribute to the controversy of these results.
- James SA, Nivol WD (1932) A study of induced malignant tertiary malaria. Proc R Soc Med 25: 1153-1186.
- Jepson AP, Banya WA, Sisay-Joof F, Hassan-King M, Bennett S, et al. (1995) Genetic regulation of fever in Plasmodium falciparum malaria in Gambian twin children. J Infect Dis 172: 316-319. [crossref]
- Abel L, Cot M, Mulder L, Carnevale P, Feingold J (1992) Segregation analysis detects a major gene controlling blood infection levels in human malaria. Am J Hum Genet 50: 1308-1317.
- Sjöberg K, Lepers JP, Raharimalala L, Larsson A, Olerup O, et al. (1992) Genetic regulation of human anti-malarial antibodies in twins. Proc Natl Acad Sci U S A 89: 2101-2104. [crossref]
- Contu L, Carcassi C, Orru S, Mulargia M, Arras M et al., (1998) HLA-B35 frequency variations correlate with malaria infection in Sardinia. Tissue Antigens 52: 452-461.
- Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, et al. (1991) Common west African HLA antigens are associated with protection from severe malaria. Nature 352: 595-600. [crossref]
- Rihet P, Traoré Y, Abel L, Aucan C, Traoré-Leroux T, et al. (1998) Malaria in humans: Plasmodium falciparum blood infection levels are linked to chromosome 5q31-q33. Am J Hum Genet 63: 498-505. [crossref]
- Traoré Y, Rihet P, Traoré-Leroux T, Aucan C, Gazin P, et al. (1999) Analysis of the genetic factors controlling malarial infection in man. Sante 9: 53-59. [crossref]
- Flori L, Kumulungui B, Aucan C, Esnault C, Traore AS, et al., (2003) Linkage and association between Plasmodium falciparum blood infection levels and chromosome 5q31-q33 Genes Immun 4: 265-268.
- WHO (2002) Roll back malaria. Regional Office for the Eastern Mediterranean 7-8.
- Douglas JT, Ott J (1994) Handbook of Human Genetic Linkage. Johns Hopkins University Press London.
- Molyneux ME (1990) The clinical features of cerebral malaria in children. Med Trop (Mars) 50: 65-68. [crossref]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11: 241-247. [crossref]
- Morton NE (1998) Significance levels in complex inheritance. Am J Hum Genet 62: 690-697. [crossref]
- Strachan T, Read P (1999) Molecular Human Genetic 2. Bios Scientific publishers Ltd: 275-277.
- Curfs JH, van der Meer JW, Sauerwein RW, Eling WM (1990) Low dosages of interleukin 1 protect mice against lethal cerebral malaria. J Exp Med 172: 1287-1291. [crossref]
- Pichyangkul S, Saengkrai P, Webster HK (1994) Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-alpha and interleukin-1 beta. Am J Trop Med Hyg 51: 430-435.
- Rockett KA, Awburn MM, Rockett EJ, Clark IA (1994) Tumor necrosis factor and interleukin-1 synergy in the context of malaria pathology. Am J Trop Med Hyg 50: 735-742. [crossref]
- Girardin E, Dayer JM (1993) Cytokines and antagonists in septic shock. Schweiz Med Wochenschr 123: 480-491.
- Huang J, Gao X, Li S, Cao Z (1997) Recruitment of IRAK to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc Natl Acad Sci U S A 94: 12829-12832. [crossref]
- Clark AG, Hall SK, Shipston MJ (1999) ATP inhibition of a mouse brain large-conductance K+ (mslo) channel variant by a mechanism independent of protein phosphorylation. J Physiol 516: 45-53.
- Weiger TM, Hermann A, Levitan IB (2002) Modulation of calcium-activated potassium channels. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188: 79-87. [crossref]
- Wallner M, Meera P, Toro L (1999) Molecular basis of fast inactivation in voltage and Ca(2+)-activated K(+) channels: a transmembrane beta-subunit homolog. Proc Nat Acad Sci 96: 4137-4142.
- Cox DH, Aldrich RW (2000) Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol 116: 411-432. [crossref]
- Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, et al. (2000) Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem 275: 23211-23218. [crossref]
- Löhn M, Lauterbach B, Haller H, Pongs O, Luft FC, et al. (2001) Beta(1)-Subunit of BK channels regulates arterial wall[Ca(2+)] and diameter in mouse cerebral arteries. J Appl Physiol (1985) 91: 1350-1354. [crossref]
- Saltman DL, Dolganov GM, Warrington JA, Wasmuth JJ (1993) A physical map of 15 loci on human chromosome 5q23-q33 by two color fluorescence in situ hybridization. Genomics 16: 726-732.
- Rihet P, Traore Y, Aucan C, Traore TL, Kumulungui B, et al., (1999) Genetic dissection of Plasmodium falciparum blood infection levels and other complex traits related to human malaria infection. Parassitologia 41: 83-87.
- Garcia A, Marquet S, Bucheton B, Hillaire D, Cot M, et al., (1998) Linkage analysis of blood Plasmodium falciparum levels: interest of the 5q31-q33 chromosome region. Am J Trop Med Hyg 58: 705-709.
- Marsh DG, Neely JD, Breazeale DR, Ghosh B, Freidhoff LR, et al., (1994) Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science 264: 1152-1156.
- Meyers DA, Postma DS, Panhuysen CI, Xu J, Amelung PJ, et al. (1994) Evidence for a locus regulating total serum IgE levels mapping to chromosome 5. Genomics 23: 464-470. [crossref]
- Gerardin P, Dorkenoo A, Cremer R, Chenaud M, Camus D, et al., (2002) Life threatening Plasmodium falciparum parasitemia, in an infant returning from the tropics. Arch Pediatr 9: 1260-1263.
- Perlmann P, Perlmann H, ElGhazali G, Blomberg MT (1999) IgE and tumor necrosis factor in malaria infection. Immunol Lett 65: 29-33. [crossref]
- Newton CR, Taylor TE, Whitten RO (1998) Pathophysiology of fatal falciparum malaria in African children. Am J Trop Med Hyg 58: 673-683. [crossref]



