Objective: to investigate the changes and potential significance of Treg (regulatory T cell) and IL-35 in rat model of acute pancreatitis in the primary 48hrs of the early developmental phase of the disease process.
Methods: 36 S-D rats were divided into two groups, control group (6 rats) and acute pancreatitis (30 rats). A rat model of pancreatitis was developed by common bile duct (CBD) ligation method. After successful development of pancreatitis model, investigations were carried out and the rats were executed by six in number each time in 2, 6, 12, 24 and 48 hours. The durations were calculated from the time of CBD ligation. CD4+ CD25+ T cells in peripheral blood were calculated by flow cytometry. Serum IL-35 was measured by ELISA method. The pathological grading of pancreatic necrosis was determined during all stages.
Results: Significant reduction of Treg and IL-35 in the AP group in 2, 6, 12, 24 and 48 hours were found in compares with the control group. Pancreatic pathology score was increased with the progression of disease.
Conclusion: According to our study regulatory T cells and IL-35 both showed a significant positive correlation with the AP group with a downward trend in their circulating level and negatively correlated with the pathological grading of the pancreas.
acute pancreatitis, pathological grading, immune changes, Treg, IL - 35
Acute pancreatitis is a severe medical condition related to high mortality and morbidity. This condition refers to a state of imbalance in host immunological stress and immunosuppressive activity. Understanding the relationship with the biochemical modulator in the pathophysiological process is extremely important for the understanding and management of diseases. T regulatory cells or Tregs and IL35 are one of the regulatory mechanisms that are responsible for the maintenance of the host the immune response and tolerance to self-antigens and autoimmune disease process. In the case of acute pancreatitis, the number of CD3+ CD4+ T cells and CD4+ CD8+ ratio reduces in the peripheral blood. During the disease process, various inflammatory mediators and cytokines are produced which leads to immunological changes, increase in infection status along with the loss of functional capacity of organs [1]. The immune system has autoregulatory capacity and modulators, among them Treg bears a negative immune feedback [2-3]. IL35 has been described for its role in immune suppression in different kinds of literature though the exact mechanism of action is still unknown [4]. In this study, we intended to investigate the potential correlation and changes of Treg and IL35 in the peripheral blood in the early phase of the rat model of acute pancreatitis.
36 S-D adult male rats, weight 200 gram in an average were collected from Jiamusi University center for the animal experiment.
Following Instruments and reagents were used
LX - 200 palm centrifuge (Haimen kirin instrument factory), FACS caliber flow cytometry analyzer (BD company, USA), CD4 antibodies, CD25 (Jin Shanqiao company), interleukin 35 rats Elisa kits (Shanghai source company), - 80 ℃ ultra-low temperature freezer (Japanese SANYO company), YBL - 2 biological tissue embedding machine, HM - 315 tissue slicing machine.
Study groups and modeling
For the study purpose the rats were randomly divided into control group (6rats) and AP (Acute pancreatitis) group (30 Rats). The AP rat models of were established by CBD (Common Bile Duct) ligation method [5]. The rats were properly fed and kept in same environmental condition. Before CBD ligation, the AP group rats were kept for 12hours of fasting. An intraperitoneal injection of Choral hydrate was given to induce anesthesia. Under all aseptic precaution, the laparotomy was performed by ventral midline incision; CBD was identified and ligated accordingly (Figure 1). The wound was closed with interrupted sutures and a sterile dressing was done. The control group was kept on Ad libitum. Pancreatic pathological grading was calculated depending on the edema, leukocyte infiltration, hemorrhage and degree of necrosis by using Jian-Xin wu grading score [6].
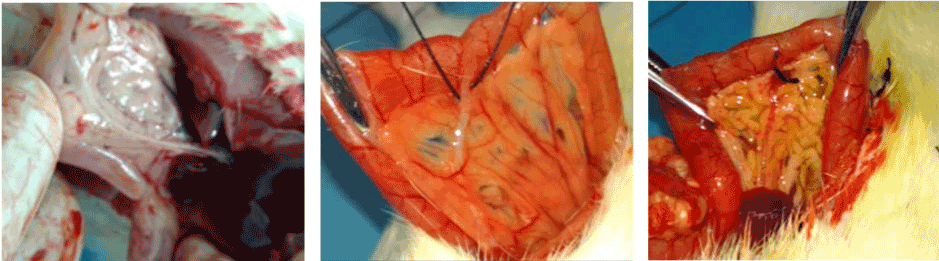
Figure 1. (A, B & C): Identification and ligation of the common bile duct.
Collection of specimens
After successful establishment of rat models, blood and tissue samples were collected from 6rats of AP group and one from the control group in each time. 5 ml blood from the inferior vena cava was collected after 2 h, 6 h, 12 h, 24 h and 48 hrs from each rat in a vacuum tube containing an anticoagulant. The duration was calculated from the time of CBD ligation. Blood samples were centrifuged in 3000RPM for 15 mins and stored in -80°C in cryo-preservation. Pancreas samples were collected and preserved in formaldehyde solution for routine paraffin embedding after 48 hours.
Pathological grading and changes in pancreatic tissue
Pancreas tissue samples were collected, preserved and prepared accordingly. Hematoxylin-eosin stain was done and the samples were observed under microscopy to identify the pathological changes. The pathological grading and scoring of the collected samples were done according to Wu Jian Xin reference [6].
Evaluation of Treg and IL - 35 levels
5 ml of blood from the inferior vena cava was collected from each rat into a vacuum tube. Then the blood samples were diluted into heparin solution and hemolysis was observed and washed, lymphocytes were separated and isolated in a 12 × 75 mm tube, mixed with CD4 and CD25 antibody (Zhongshan Jinqiao) and incubated in dark for 20mins. Then the tubes were placed into the flow cytometer machine. Results were obtained for CD4+CD25+Treg as a mean ± standard deviation. IL-35 was measured by standard enzyme-linked immunosorbent assay.
SPSS17.0 was used for statistical analysis. Chi-square test and Pearson correlation analysis were done. A P value of < 0.05 was considered significant.
Morphological and microscopical changes in pancreatic tissue
No significant changes were noticed in the control group. (Figure 2a) Under microscopy, the acinar lobules were intact with clear stroma and mild interstitial edema. (Figure 2b & 2c) Microscopical evidence of hemorrhage, necrosis, and abundant interstitial mononuclear inflammatory cells was observed (Figure 3). In AP Group, the acinar lobular structure was found loose and distorted filled with pancreatic interstitial edematic fluid (Figure 4-8). Pathological changes of pancreas increased time after modeling. The results are shown in Table-1. Changes in Treg and IL - 35 levels in peripheral blood and their correlation with pathologic grading of pancreas:
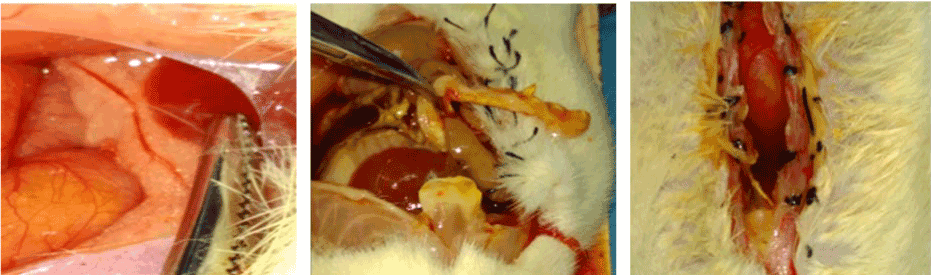
Figure 2. (A, B & C): Morphological Change of pancreas and peritoneum following modeling. A: normal pancreas, B & C: Changes of pancreas and peritoneum following CBD ligation.
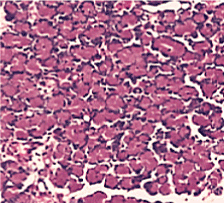
Figure 3. Normal control group (HE stain × 400)
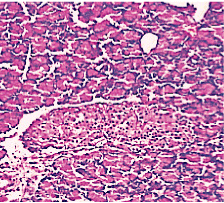
Figure 4. AP model group 2h (HE staining × 400) mild Lymphocyte infiltration and primary edema.
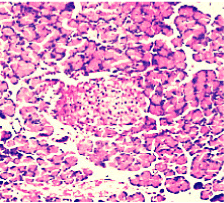
Figure 5. AP model group 6h (HE staining × 400) moderate lymphocyte infiltration.
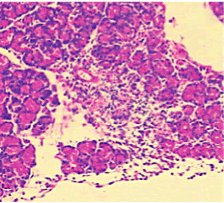
Figure 6. AP model group 12h (HE staining × 400) marked infiltration of lymphocyte in the pancreatic acinar cells with gross oedematic change.
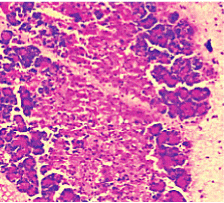
Figure 7. AP model group 24h (HE staining × 400) partial loss of acini structure and gross oedema.
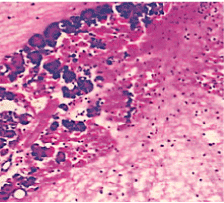
Figure 8. AP model group 48 h (HE staining × 400) complete loss of acini architecture.
In the control group blood Treg was found 2.706% while in the AP group the average value was less than 2%. 2 hours after modeling in AP group this value was 1.735% on an average. With time, the level declined and the lowest value was obtained 0.744% in an average at 48hours (Figure 9-13). The statistical analysis showed a significant reduction P <0.05 [Table 1].
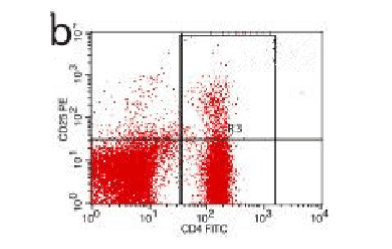
Figure 9. AP model group 2h
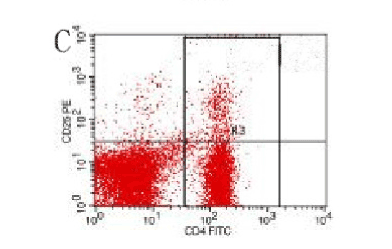
Figure 10. AP model group, 6 h
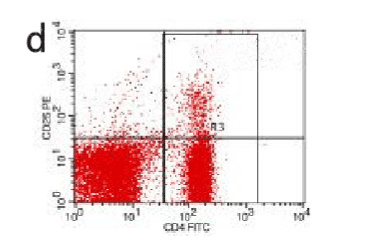
Figure 11. AP model group 12h
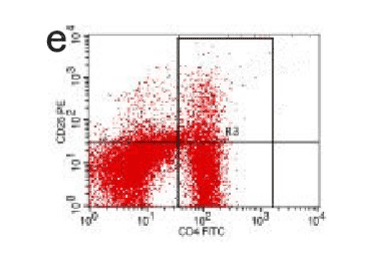
Figure 12. AP model group 24h
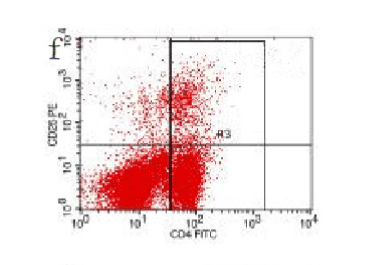
Figure 13. AP model group 48h
IL-35 level in the control group was 1.04 ± 0.12 pg/ml while in the experimental group the level was 0.74 ± 0.17 at 2 hours. This value gradually decreased and at 48 hrs the up to the lowest 0.31 ± 0.11 pg/ml (Table 1).
Table 1. Changes in Treg and IL-35 in the peripheral blood. Pathological grading (X ± S) of the pancreas in the study groups. (Note: Values were compared with control group, P < 0.05, *P < 0.05, ^ P < 0.05).
Group |
Treg (%) |
IL-35 (pg/ml) The pancreas pathological grading |
The control group |
2.71 ± 0.14 |
1.04 ± 0.12 0.00 ± 0.00 |
SAP2h |
1.74 ± 0.17# |
0.74 ± 0.17* 4.66 ± 1.37^ |
SAP6h |
1.29 ± 0.16# |
0.66 ± 0.43* 6.83 ± 1.33^ |
SAP12h |
1.10 ± 0.06# |
0.59 ± 0.59* 10.50 ± 1.38^ |
SAP24h |
1.05 ± 0.02# |
0.39 ± 0.33* 13.00 ± 1.41^ |
SAP48h |
0.74 ± 0.15# |
0.31 ± 0.11* 13.83 ± 1.70^ |
Pathological grading under bright light naked eye examination and Microscopy revealed a maximum - minimum value 3.23 to 15.06 from 2 h to 48 hours. This showed a continuous rising pattern.
Serum Treg level and IL-35 level was positively correlated (Pearson correlation = 0.398, P < 0.05). This was negative with pancreatic pathology score for both types (Pearson correlation = 0.869 and 0.407, P < 0.05).
Acute pancreatitis can be triggered by a variety of pathophysiological mechanisms, among this impaired immune system plays a certain important role. In this study, we tried to evaluate the relationship between the developmental phase of pancreatic inflammation with the changes of Treg and IL35 level in the circulation. Our findings reveal that in the experimental group (AP) blood level of Treg gradually decreased from 2hrs up to 48hrs (P<0.05) while at the same time pancreatic pathology score increased gradually. This proved a significant association between the Treg and pancreatic pathological changes in case of acute pancreatitis. CD4, CD25, and Treg are immunological mediators with negative feedback effect. Studies have described such immune inhibition in a variety of ways, among which IL-2 pathway plays the most significant role [7]. The proliferation of T-lymphocyte is influenced by IL-2, Treg, and CD25. IL-2 receptor alpha chain competes for the receptor binding site in the effectors cells with each other. The dominant role of Treg cells results in decreased IL2 and cytokines; this ultimately inhibits the rapid growth of T cells. In the initial phase of acute pancreatitis, pro-inflammatory response plays a dominant role. As the disease progress, the pro and anti-inflammatory response gradually inhibits the immunological reaction and the IL-2 level decreases. This mechanism results in gradual activation of T lymphocytes. In our current study, the AP group the percentage Treg shared a close outline with the control group till 2 hrs, (Figure 14) but this level gradually reduced with time compare with the control group (Figure 8-12). Thereby this finding indicates a potential role of reduced Treg cell level with excessive immune activation in case of the initial phase of acute pancreatitis in rats. The similar findings were described by Ding Zhen [8] and several other kinds of literature. A study performed by Dante's Peak described a significant fall in The Treg level after 6hrs of modeling. Besides this, our study revealed a significant correlation with IL-35 level with acute pancreatitis. IL-35 level significantly decreased (P<0.05) with time as the pancreas pathology score rise in the rat models of AP group. This finding confirms the recent understanding of the negative effect of IL-35 on the lymphocyte proliferation and the expression of inflammatory cytokines [9,10]. Additionally, iTreg cell activated by CD4, CD25, and Treg can secrete IL-35, which intern has a positive effect on iTreg [2]. In the initial phase of acute pancreatitis, the host immune response causes a negative effect on inflammation. But in the later phase due to increasing activity of pro-inflammatory cytokines, the inhibitory responses become suppressed and this is expressed by the significant reduction of circulation IL-35 level with time.
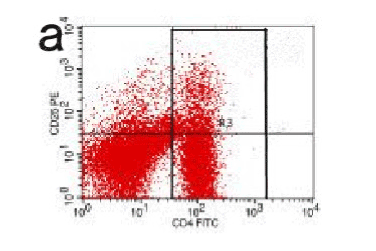
Figure 14. CD
In conclusion, according to our study, serum IL-35 and Treg levels have a significant potential role with the developmental phase of acute pancreatitis in the rat model. This finding promotes an accurate and comprehensive understanding of the Pathophysiology of acute pancreatitis. We believe this finding will play an effective guideline in the treatment and outcome of acute pancreatitis.
Authors of this manuscript declare no conflict of interest.
Authors would like to thank all the doctors and staffs of Department of Digestive Disease-II and Prof (Dr) A S M Mostaque Ahmed, Principle “Chattagram Maa-O-Shishu Medical College” for their generous cooperation and support. The study was approved by the local ethical committee of First affiliated hospital of Jiamusi University and was supported by project fund: Innovation and Technology Graduate subject Jiamusi University, NO: LM2015_025.
- Kylanpaa ML, Repo H, Puolakkainen PA (2010) Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol 16: 2867-2872. [Crossref]
- Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, et al. (2010) IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 11: 1093-1101. [Crossref]
- Sakaguchi S (2011) Regulatory T cells: history and perspective. Methods Mol Biol 707: 3-17. [Crossref]
- Luo Cheng, Ji Qing Wei, Lin Ying Zhong (2012) The structure of interleukin - 35 and its relation with disease research progress. Chinese Journal of Cellular and Molecular Immunology 28: 769-770
- Tang Haotao, Xue Xing (2014) The establishment of the experimental animal model of acute pancreatitis methods and research progress [J]. Chinese Journal of Current Advances in General Surgery 17: 412-414
- Wu Jian Xin, Yuan Yao Zong, Xu Jia Yu (2002) Type in rats with acute necrosis pancreatitis pathological characteristics research of evaluation methods [J]. Acta Laboratorium Animalis Scientia Sinica10: 210-212
- Buckner JH (2010) Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 10: 849-859. [Crossref]
- Ding Zhen, Lin Rong, Qian Wei (2007) CD4+ CD25+ cells and incidence of the early immune necrotizing pancreatitis in rats [J]. Chinese Journal of Digestion 27: 585-587
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, et al. (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450: 566-569. [Crossref]
- Liu F, Tong F, He Y, Liu H (2011) Detectable expression of IL-35 in CD4+ T cells from peripheral blood of chronic hepatitis B patients. Clin Immunol 139: 1-5. [Crossref]














