Abstract
Pickering emulsions are fine dispersions of minute droplets of two immiscible phases stabilized by solid particles adsorbed at the liquid-liquid interphase. In the case of oil-in-water (o/w) or water-in-oil (w/o) emulsions, such types of Pickering emulsions have utility in various applications such as the preparation of porous materials due to their ability to self-assemble at the o/w interface. Iron oxide nanoparticles (Fe3O4 NPs) are known to play an important role in stabilizing Pickering emulsions due to their variable functionality and hydrophobicity. The efficiency of Fe3O4 NPs in stabilizing Pickering emulsions depend on the polarity of the oil used in the preparation of the Pickering emulsion. Fe3O4 NPs can be surface modified through coordination via carboxylic acids and silane coupling agents. Non-modified and carboxylic acid-modified Fe3O4 NPs provide greater stability for o/w emulsions prepared with non-polar or weakly polar oils. Silane-coated Fe3O4 NPs are more useful in stabilizing o/w emulsions where the oil phase is strongly polar. The chain length of the modifying agent plays an important role on the stability of emulsions. Modifying agents with long alkyl chains result in greater hydrophobicity that adversely affect the stability of such Pickering emulsions. Other factors governing the stability of these emulsions include the pH of the aqueous phase, oil polarity, concentration/size of particles, ion concentration as well as oil/water volume ratio. A key parameter used for determining the effectiveness of particle stabilization of Pickering emulsions is the contact angle at the o/w interface. Contact angle (q) measurements provide a measure of the particle hydrophobicity, while qvalues provide a measure of colloidal stabilization of such particles. The aim of this review is to provide an overview of the effectiveness of Fe3O4 NPs as stabilizing agents for Pickering emulsions and to bridge the knowledge gaps related to this topic in the last 5 years. This review describes the utility of iron oxide NPs as emulsion stabilizers by outlining aspects of the structure and the physicochemical properties of such systems. The use of surface modified Fe3O4 NPs as stabilizing agents is anticipated to grow, where the results of this overview will catalyze further studies directed at advanced research and development of such systems for diverse applications in materials science, colloid chemistry and medicine.
Keywords
pickering emulsion; iron oxide; nano-particles; stabilization; interphase; contact angle
Introduction
Emulsions are defined as fine dispersions of minute droplets of one liquid in another immiscible liquid which are often formed either spontaneously or due to mechanical agitation. Emulsions are made up of the continuous and dispersed phases, where the dispersed phase in the form of a droplet is typically suspended in the continuous phase. Emulsions may be classified into: i) oil-in-water (o/w) ii) water-in-oil (w/o) and iii) complex emulsions [1-3]. The high interfacial energy of the dispersed droplets in the emulsion phase contributes thermodynamic instability. Therefore, stable emulsions can be obtained using substances known as emulsifiers which are able to decrease the interfacial tension between the phases and are usually included in emulsion formulations [2]. An emulsifier is described as chemical species with amphiphilic properties that adsorbs strongly at the oil/water (o/w) interface. The adsorption of the emulsifiers at this interface lead to the formation of a protective film which resist droplet coalescence and phase separation, thus aiding emulsion stabilization. Conventional emulsifiers include low molar mass surfactants such as sodium dodecyl sulfate or surface-active polymers [2,4].
In 1903 and 1907, the studies of Ramsden [5] and Pickering [6] revealed that solid particles are able to absorb at the oil–water interface and are suitable for stabilizing emulsions of oil and water [2,4,7]. The particle-stabilized emulsions were henceforth named Pickering emulsions after S. U. Pickering, even though the effect was first recognized by Walter Ramsden. The low toxicity and superior stability of Pickering emulsions lend unique properties when compared against classical emulsions stabilized by surfactants. Several studies on particle-stabilized emulsions have shown that thermodynamic and kinetically stable Pickering emulsions were prepared using nanoparticles (NPs) from various materials such as gold [8], biomaterials [9], latex [10], carbonyl iron [7], modified and non-modified iron oxide [11-14], silica [10], titanium dioxide [15] and zinc oxide [16]. The characteristics of these particles that contribute uniquely relative to conventional emulsifiers include irreversible interfacial adsorption, stability against coalescence, sedimentation, flocculation and creaming, along with the ability to stabilize emulsions with large droplet size (up to several millimeters) [17].
Among the various types of NPs used for the stabilization of Pickering emulsions mentioned above, iron oxide (Fe3O4) NPs have generated great interest due to their magnetic properties and biocompatibility [18,19]. Previous studies (Table 1) on the use of Fe3O4 NPs as stabilizers for Pickering emulsions have shown that modified [12-14] as well as non-modified Fe3O4 NPs [11] are suitable for stabilizing Pickering emulsions. The modification of Fe3O4 NPs may be achieved via coating with carboxylic acids [4,12,14] silane coupling agents [12,20], polystyrene [21] and graphene oxide [22]. Non-modified and carboxylic acid-modified Fe3O4 NPs provide greater stability for o/w emulsions of non-polar or weakly polar oils. By comparison, silane-coated Fe3O4 NPs are better stabilizers for o/w emulsions whose oil component is both non-polar and strongly polar [14,20,23]. The unique properties of magnetic-based Pickering emulsions include greater stability and biocompatibility which allow usage for encapsulation, chemotherapy, in vivo Magnetic Resonance Imaging (MRI), genomics and theranomics applications [14,24-27]. This study presents an overview of the factors affecting the stability of Fe3O4 NPs stabilized Pickering emulsions. This overview will provide a greater understanding of the effectiveness and drawbacks of Fe3O4 NPs as stabilizing agents for Pickering emulsions and to provide a bridge to address the knowledge gaps in this field over the last 5 years [9,28-34].
Particle |
Modifier |
Emulsion type |
References |
Fe3O4 NP |
Stearoyl lactylate |
o/w and w/o |
[4] |
Fe3O4 NP |
Oleic acid |
o/w |
[32] |
Fe3O4 NP |
|
w/o |
[11] |
Fe3O4 NP |
Silane coupling agents/ carboxylic acids |
o/w |
[23] |
Fe3O4 NP |
Oleic acid |
o/w |
[13] |
Fe3O4 NP |
Oleic acid |
o/w |
[14] |
Carbonyl iron |
NA |
o/w |
[7] |
FeOx |
polystyrene |
o/w |
[21] |
Fe3O4 NP |
Carboxylic acid |
w/o |
[33] |
Fe3O4 NP |
Graphene oxide |
w/o |
[34] |
Fe3O4 NP |
Silane coupling agent |
o/w |
[20] |
Table 1: Summary of selected studies on Fe3O4 NP stabilized emulsions.
Types of Pickering Emulsions
Pickering emulsions can be classified into three general classes as follows: i) oil-in-water (o/w), ii) water-in-oil (w/o) emulsions and iii) complex emulsions.
Oil-in-water (o/w) Pickering emulsions
Oil-in-water Pickering emulsion refers to an emulsion where droplets of oil are dispersed in water as the continuous phase (Scheme 1). This type of Pickering emulsion is generally stabilized by NPs whose contact angle is less than 90o. Previous studies have reported the use of modified and non-modified Fe3O4 NPs for the stabilization of Pickering emulsion systems containing dodecane, octadecenyl succinic anhydride (ODSA), toluene and butyl butyrate in water, respectively [4,11,35].
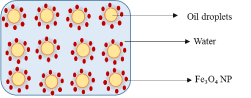
Scheme 1: Schematic presentation of stabilization of o/w Pickering emulsion by iron oxide
Water-in-oil (w/o) Pickering emulsions
Water-in-oil Pickering emulsions contain droplets of water as the dispersed phase and oil as the continuous phase (Scheme 2). NPs with contact angle greater than 90o are the best stabilizing agents for these types of emulsions. Liu et. al [36] have reported the stabilization of o/w Pickering emulsions by magnetic NPs
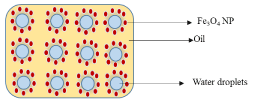
Scheme 2: Conceptual stabilization of w/o Pickering emulsions by iron oxide NPs
Complex Pickering emulsions
Multiple Pickering emulsions are complex polydisperse systems where tiny droplets are suspended in bigger droplets that are also suspended in a continuous phase and they exist simultaneously. In this type of Pickering emulsion (w/o/w), the primary w/o emulsion is stabilized by hydrophobic nanoparticles; whereas, hydrophilic nanoparticles are used as stabilizers in the secondary emulsification process. Complex emulsions can be in the form of water-in-oil-in-water (w/o/w) or oil-in-water-in-oil (o/w/o) (Scheme 3), [37].
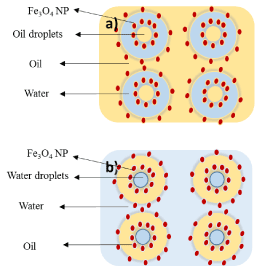
Scheme3: Illustrative view of the distribution and stabilization of complex Pickering emulsion by iron oxide (a) o/w/o, (b) w/o/w
Preparation of Pickering emulsions
Different types of Pickering emulsions can be prepared in a manner similar to classical surfactant-based emulsions. Typically, the stabilizing agent is first solvated in the primary liquid with mechanical agitation followed by the addition of the second liquid. For example, o/w emulsions are prepared by wetting hydrophilic nanoparticles in the aqueous phase (primary liquid) with mechanical agitation using gentle magnetic stirring or ultra-sonication. This is followed by emulsification of the oil phase using a suitable process that depends on the required droplet size. On the other hand, a w/o emulsions can be prepared as described above, where a key difference relates to the initial wetting of the hydrophobic stabilizing agent in the oil phase. For complex emulsions such as a w/o/w, the primary w/o emulsion is stabilized by hydrophobic NPs followed with dispersion of the primary emulsion in water using hydrophilic NPs as emulsifiers. Generally, the nature of agitation after addition of the primary and secondary phases, along with the size and nature of particles, affect the droplet size of the resulting emulsion (Table 2 and Figure 4).
Table 2: Preparation of Pickering emulsions*
Emulsion type/
droplet size |
Types
of nanoparticles |
Process |
o/w emulsion
(< 1μm) |
Hydrophilic NPs |
- Dispersion of NPs in the water phase.
- Emulsification of the oil phase using ultrasound or high pressure homogenizer.
|
From 1μm to 10 μm |
Hydrophilic nano- or sub-micron sized particles |
- Dispersion of particles in water.
- Emulsification of the oil phase using a high speed rotor stator device.
|
From 10μm to 100 μm |
Hydrophilic micro- or sub-micron sized particles |
- Dispersion of particles in water.
- Emulsification of the oil phase using a rotating propeller stirrer.
|
Above 100 μm |
Hydrophilic micro particles |
- Dispersion of particles in water.
- Emulsification of the oil phase by means of gentle mixing: hand shaking or rotating blade stirrer.
|
w/o emulsion |
Hydrophobic particles |
1. Dispersion of particles in oil phase.
2. Emulsification of the water phase by means of appropriate technology with respect to the desired droplet size. |
Complex (w/o/w) emulsion |
Hydrophobic particles for the primary w/o emulsion and hydrophilic particle for the secondary emulsion |
- Preparation of the primary w/o emulsion.
- Dispersion of the particles in the oil phase.
- Emulsification of the water phase by means of appropriate technology with respect to the desired droplet size.
- Preparation of the w/o/w emulsion.
- Dispersion of the particles in the aqueous phase.
- Emulsification of the primary emulsion into the aqueous phase under low shear.
|
* Adapted from Chevalier and Bolzinger, 2013[29]
For example, dispersion of the primary liquid with ultra-sonication yields fine suspension of particles with a 100 nm mean diameter, while high speed (10,000–20,000 rpm) mechanical stirring (using Ultra-Turrax) results in particles ca. 2μm diameter. Also, emulsification of the secondary liquid with an Ultra-Turrax rotor stator device rotating at 10,000–20,000 rpm (Figure 1) for 10 mins. Yield droplets with an average diameter ca. 2–5 μm range (Figure 2), depending on the viscosity of the liquid [11,20,29,38].
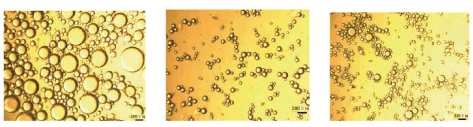
Figure 2: Microscope images showing different emulsion droplet sizes.Reproduced with Permission [11]

Figure 1: Ultra-Turrax rotor stator device. Adapted from the following source: https://profilab24.com/Dispersers-Homogenizers-High-Speed-Mixers [38].
Stabilization of Pickering emulsion by iron oxide nanoparticles (Fe3O4 NPs)
Fe3O4 NPs are iron oxide that exhibit particle diameters between 1 to 100 nm [39]. These NPs have attracted significant interest due to their unique properties which afford their potential applications in biosensors, magnetic resonance imaging (MRI) contrast agents, emulsifiers and magnetic fluid hyperthermia. These properties include high surface area, benign nature, and amenability to surface modification, negligible toxicity, biocompatibility, biodegradability and physical/chemical stability. Among the properties listed above, the relatively high surface area and facile surface modification enable favorable adsorption at the o/w interface. Thus, Fe3O4 NPs are suitable stabilizing agents, where the application of modified and non-modified NPs was previously reported as Pickering emulsion stabilizing agents [11-14,40]. These studies highlighted that variable factors affect the stabilization of Pickering emulsions by Fe3O4 NPs.
It is worthwhile to note that some challenges exist for the effective application of Fe3O4 NPs as emulsion stabilizers. These include but are not limited to NPs with controlled size, shape, stability, and the inability to be dispersed in certain types of solvents. The tendency of NPs to undergo aggregation is related to the minimization of the surface energy. Large surface energy is a consequence of their large surface to volume (S/V) ratio. The unmodified Fe3O4 NPs are easily oxidized due to their high chemical activity. One method to overcome these challenges involves surface modification of the NPs with a suitable organic or inorganic moiety. Surface modification not only stabilizes the Fe3O4 NPs, but it can lead to further fictionalization of the particles [41].
Factors affecting the stability of Pickering emulsions
The Wettability of Fe3O4 NPs at the o/w interface plays a critical role in the emulsification efficacy of these particles. Particle Wettability can be characterized using the contact angle (θ) at the oil-particle-water interface (Scheme 4). Hydrophilic particles usually exhibit θ < 90°; whereas, hydrophobic particles are characterized by a greater contact angle (θ > 90°) with water. According to equation 1, particles with θ = 90° at the oil/water interface possess the maximum desorption energy and are generally not suitable for stabilizing Pickering emulsions [2].
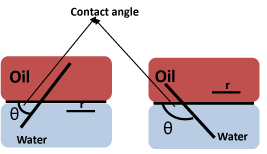
Scheme 4: Contact angles (θ) at the oil/water interphase where r is the radius of the particle, where r above refers to R in eqn 1.
E = pR2gow (1+ cos θ2) Eqn 1
gow represents the tension of the o/w interface and R represents the radius of a single spherical particle [2]. The factors that influence the wetting properties of NPs at the o/w interface are discussed in the section below.
Effects of Modification of Fe3O4 NPs
Among the factors influencing the stability of Pickering emulsions, hydrophobicity/hydrophilicity of the particles is a key parameter since it determines the Wettability of the NPs at the o/w interphase. One way of tuning the hydrophobicity and hydrophilicity of NPs is surface modification using carboxylic acids, silane coupling agents and polystyrene, etc. Zhou et. al.[11] Reported that non-modified and carboxylic acid-modified Fe3O4 NPs were shown to provide greater stability for o/w emulsions using non-polar or weakly polar oils. By contrast, silane-coated Fe3O4 NPs are more useful in stabilizing o/w emulsions whose oil component is strongly polar. They also reported that the chain length of the carboxylic acid modifier influences the stabilizing efficiency of the resulting Fe3O4 NPs. Modification of the NPs by enhancingthe hydrophobicity occurs by decreasing the relative percentage of hydroxyl groups on the NP surface. Ingram et. al.[13] and Lan et. al.[14] reported the use Fe3O4 NPs coated with oleic acid bilayers (cf. Fig. 3a) for the stabilization of Pickering emulsions. Vengsarkar [4] also reported the stabilization of Pickering emulsions using monolayer and bilayer stearoyl lactylate coated Fe3O4 NPs (Figure 4).
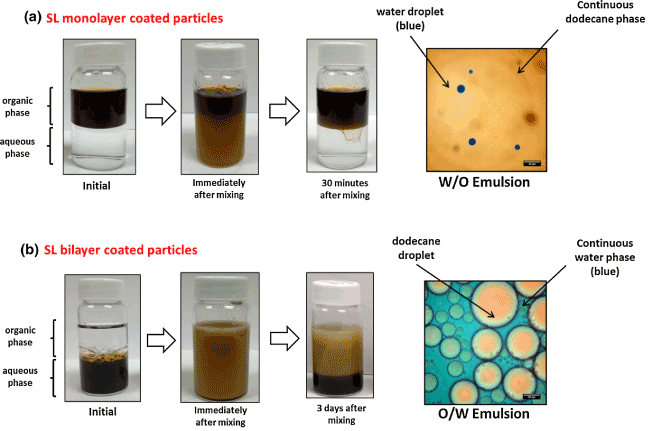
Figure 4: Stabilization of Pickering emulsion by a) SL monolayer and b) SL bilayer coated iron oxide NPs. (pH of aqueous phase = 10 and iron oxide NP concentration = 0.1 wt %).Reproduced with Permission [4].
The study revealed that the monolayer coated Fe3O4 NPs (Figure 3b) generated extremely unstable w/o emulsions; whereas, the bilayer coated Fe3O4 NPs successfully stabilized an o/w emulsion. The study carried out by Kaiser et. al.[21] Showed that polystyrene coated Fe3O4 NPs displayed good emulsion stability due to entanglement of the polymer shell. In another report, Qiao et. al.[20] Discovered that silane coated Fe3O4 NPs were able to stabilize emulsions of both dodecane and butyl butyrate. They reported that despite the appearance of emulsion creaming, the systems reached a stable state by approximately 500 minutes, except for the butyl butyrate-in-water emulsion which showed a continual gradual decrease in the residual emulsion fraction.
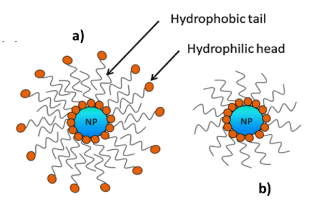
Figure 3: Structures of a) bilayer oleic acid coated Fe3O4 NPs and b) monolayer coated Fe3O4 NPs. Reproduced withPermission [4]
pH of aqueous solution
The pH of the aqueous solution is another factor that influences the emulsion stabilization capacity of Fe3O4 NPs. This relates to a change in pH due to its effect on the surface chemistry of the particles according to their respective pH of point-of-zero net charge (PNZC). Studies [4,13,14] directed at the influence of pH on the emulsification capacity of Fe3O4 NPs modified with oleic acid and stearoyl lactylate show that the zeta potential of modified NPs increased with increasing pH except after pH 10 (Figure 5).
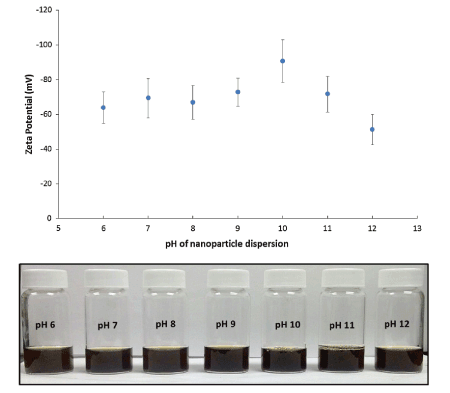
Figure 5: Stability analysis using zeta-potential of SL bilayer coated iron oxide nanoparticles at various pH values. Reproduced with Permission [4].
With the use of optical microscopy, Vengsarkar [4] revealed that alkaline emulsifying conditions (pH ≥ 9) result in o/w emulsionswith oil droplet sizes of approximately 20 µm. By contrast, reduced pH conditions (pH ≤ 8) result in a droplet size of ca. 35 µm (Figure 6). The greater droplet size for the samples at the lower pH provide support that the particles exhibit better emulsion stabilizing capacity relative to pH values between 8 and 10. The improvement in emulsion stabilization capacity of the modified Fe3O4 NPs was related to cooperative stabilization by the modifiers and the NP domains of the emulsifier. Lan et. al [14] also studied the influence of pH on Pickering emulsions stabilized by oleic acid coated Fe3O4 NPs. They reported variable long-term stability of Pickering emulsions at different pH values. They showed that acidic pH conditions (3.80 <pH <6.80) favoured the formation of w/o emulsions while alkaline pH conditions (8.40 <pH <11.30) supported the stabilization of o/w emulsions by the modified NPs. They also reported the phase inversion of Pickering emulsions at pH values 2.00 <pH <12.05.
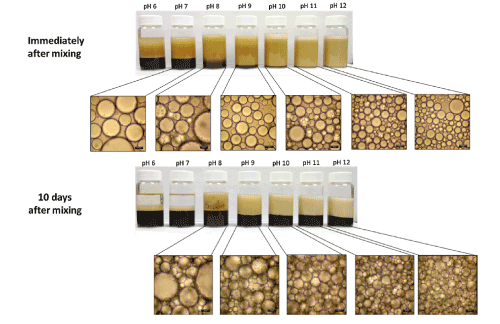
Figure 6: pH-responsiveness studies on oil-in-water Pickering emulsions generated using SL bilayer coated iron oxide nanoparticles. Reproduced with Permission [4].
Concentration/size of nanoparticles (NPs)
Zhou et al.[11] reported a study on the influence of particle concentration on the stability of Fe3O4 NPs stabilized Pickering emulsions. They showed that the concentration of NPs had no apparent effect on the stability of the prepared emulsions. The results also showed that the average droplet size decreased with an increase in particle concentration except for 0.5 wt % (Figure 7).
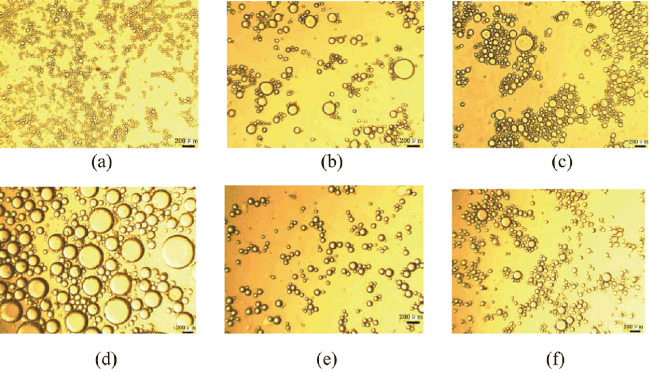
Figure 7: Microscope images 20 days after the preparation of dodecane-in-water Pickering emulsions stabilized by Fe3O4 nanoparticles at different volume fraction (Øo) values and particle concentrations: (a) 0.2, 1 wt %; (b) 0.4, 1 wt %; (c) 0.6, 1 wt %; (d) 0.8, 1 wt %; (e) 0.8, 0.5 wt %; and (f) 0.8, 2 wt %. Reproduced with Permission [11]
Binks and Rodrigues [42] reported that multiple w/o/w emulsions were formed at low particle concentrations, but simple o/w emulsions are preferred near and above 1 wt % in water for silica particle- stabilized Pickering emulsions containing a triglyceride oil. Binks and Lumsdon [43] reported on the stabilization of oil-in-water emulsions by using monodispersed latex particles. The average emulsion drop diameter (35-75 μm) increased initially with increasing particle size and then remained constant thereafter, in agreement with another independent report [10].
Oil polarity
Studies [11,12,14,23] on the effect of oil polarity on the stability of the resulting Pickering emulsion systems have shown that oil polarity influences the Wettability of Fe3O4 NPs at the o/w interface. The reports show that the non-modified and carboxylic acid modified Fe3O4 NPs exhibit good stabilization efficiency for emulsions prepared with weakly polar and non-polar oils (Figure 8). By comparison, hydrophobic particles such as silane-coated Fe3O4 NPs are very efficient in stabilizing Pickering emulsions of highly polar o/w systems.
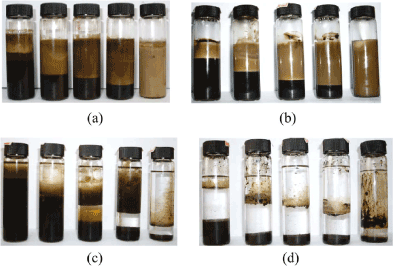
Figure 8: Photographs of the vessels containing magnetic Pickering O/W emulsions stabilized by 1 wt % Fe3O4NPs initially in water taken 20 days after emulsion preparation. From left to right, Øo = 0.2, 0.4, 0.5, 0.6, and 0.8: (a) dodecane, (b) 10 mPa s PDMS, (c) butyl butyrate, and (d) decanol. Reproduced with Permission [11]
Oil/water volume ratio
The influence of oil/water volume fraction on the stability of Pickering emulsions for variable oil types was studied by Zhou et al.[11] The results show that the residual emulsion fraction increased as the oil/water volume fraction increased. After 500 and 1000 minutes, respectively, the emulsion with oil/water volume fraction of 0.8 containing dodecane or polydimethylsiloxane (PMDS) as the oil phase was completely stable (Figure 9).
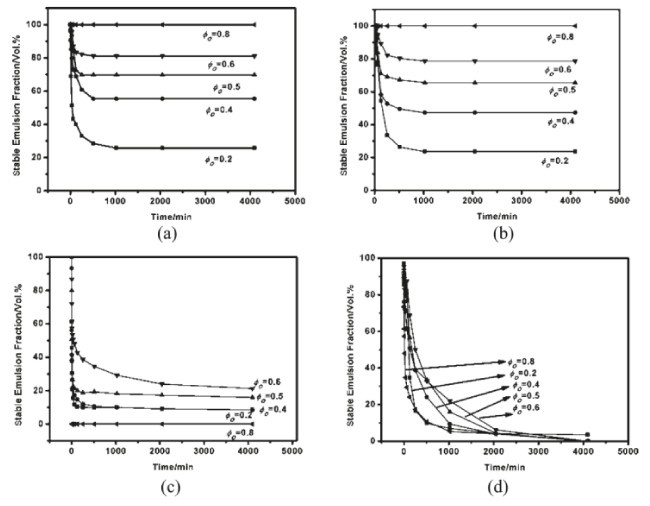
Figure 9: Change in the volume fraction of the residual emulsion vs time for O/W emulsions stabilized by 1 wt % Fe3O4NPs initially in waterwith (a) dodecane, (b) 10 mPa s PDMS, (c) butyl butyrate, and (d) decanol. Reproduced with Permission [11].
However, the butyl butyrate-water and decanol-water emulsion systems were completely unstable over the range of oil/water volume fractions studied. The foregoing provides support that dodecane-water and PDMS-water systems can form emulsions that resist coalescence due to the adsorption of Fe3O4 NPs at the o/w interface. Binks and Rodrigues [42] have demonstrated that the average drop size increased while the polydispersity decreased as the volume fraction of oil increased at a fixed particle concentration in water for o/w emulsions (Figure 10). They also reported that when particles were initially wetted with low water content relative to the oil fraction, the corresponding emulsions underwent a phase inversion from simple w/o to o/w.

Figure 10: Microscope images 20 days after preparation of butyl butyrate-in-water Pickering emulsions stabilized (c) 1% wt. 0.01OS-Fe3O4, 1:1, (d) 1% wt. 0.01OS-Fe3O4 1:2. Reproduced with Permission [12].
Effects of ion concentration
Various reports have revealed variable effects of ionic strength on the stability of Pickering emulsions [4,14]. The variation in emulsion stability was dependent on the nature of the modifier used for coating of the Fe3O4 NPs. For example, Lan et. al [14] reported the change in contact angle and zeta potential of oleic acid bilayer coated Fe3O4 NPs as the ionic strength increased. They further reported the formation of o/w emulsions when [NaCl] <0.2 M and w/o emulsions when [NaCl] >0.2 M. The emulsions were very stable to creaming and show coalescence over a wide range of NaCl concentration (0.10 M <[NaCl] <0.62 M), irrespective of the type of the emulsion. On the other hand, over the range of salt concentration, 0.03 M <[NaCl] <0.1 M and 0.62 M <[NaCl] <2.0 M, the emulsions were stable to coalescence but not creaming. Outside the range of concentrations described above, the emulsions were unstable to coalescence and creaming. The studies also showed that the size of emulsion droplets at pH 10.70 initially decreased before increasing with an increase in ionic strength. In another study, Vengsarkar [4] reported that an ionic strength of 0.1 to 3.5 wt% had no measureable effect on the stability of emulsions stabilized by Fe3O4 NPs coated with stearoyl stearate (Figure 11). Creaming was observed after 10 days in the samples, resulting in a decrease in the volume fraction of the residual emulsion to around 50%. An optical microscopy study of the samples revealed that for samples with an ionic strength > 0.1%, the size of the emulsion droplets increased (> 50 μm) due to coalescence.
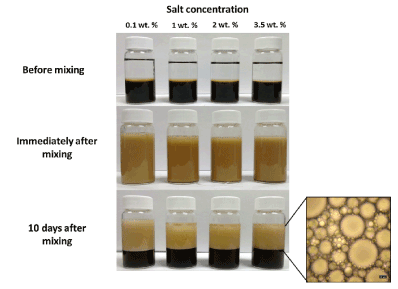
Figure 11: Effect of pH on the stability of Pickering emulsions generated using SL bilayer coated iron oxide NPs. (pH of aqueous phase before salt addition = 7.5 and iron oxide NP concentration = 0.1 wt %). Reproduced with Permission [4]
Assessment of stability of Pickering emulsions
Generally, emulsions are thermodynamically unstable due to the large interfacial area between water and oil upon emulsification. The instability is also related to the corresponding increase in the interfacial Gibbs free energy. In principle, this means that all emulsions tend to phase separate. Surfactant-based emulsifiers lower the Gibbs energy upon emulsification by reducing the interfacial tension of the oil/water interphase. On the other hand, solid particles do stabilize emulsions by adsorbing strongly at the w/o interface by reducing the surface area of the oil/water interface. The surface free energy dGσis related to three components: an entropy term SσdT, an interfacial energy term Adγ, and a composition term å nidμi (ni is the number of moles of component i with chemical potential μi), according to the Gibbs–Duhem equation
dGσ= −SσdT + Adγ+ å nidμi. Eqn. 2
At constant temperature and composition,
dGσ= Adγ Eqn. 3
therefore,γ= (Gσ/dA) T, ni Eqn. 4
Fe3O4 NPs possess high surface-to-volume (S/V) ratio which culminates in large surface energies. According to equation 3, in order to decrease their S/Vratio as well as their surface energies, the particles tend to aggregate insolvents [41]. This challenge can be minimised by surface modification of the NPs and facilitate further fictionalization of the particle surface groups. Thermodynamic studies provide information on processes taking place before and after the emulsification process. By contrast, kinetic studies afford the determination of the rate at which these processes occur. During the preparation of a Pickering emulsion, wetting of NPs in the primary phase and addition of the secondary phase results in emulsion formation. After a certain time, de-emulsification may occur which is characterized by aggregate formation and/or phase separation, where distinct layers of oil and water are visible. The phenomena of aggregate formation and phase separation are due to thermodynamic instability, while the time taken for the distinct layers to be visible relate to kinetic instability factors [30]. The thermodynamic and kinetic stability of Pickering emulsions may be assessed further, as discussed below.
Aggregate formation
Aggregate formation often occurs in the form of coalescence and flocculation and generally takes place because the emulsion droplets are always in motion (Brownian motion) and undergo collision. This motion usually induces aggregation or separation of the droplets. Flocculation is the aggregation of droplets with the droplets still maintaining their physical properties, while coalescence is characterized by the aggregation of droplets. During coalescence, the droplets eventually merge together to form larger droplets (Figure 12 and Scheme 5). Coalescence and flocculation of emulsion droplets may be assessed using methods that characterize droplet size such as microscopy and light scattering [7,11,14]. The rate of flocculation can be determined by measuring the floc size dynamically as a function of time.
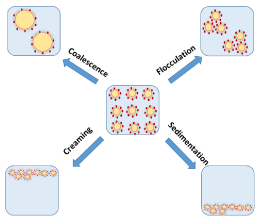
Scheme 5: Illustration of the factors that cause instability of Pickering emulsions. The blue region represents the water phase, yellow ovals represent oil droplets and red dots represent the stabilizing agent.

Figure 12: Creaming of Fe3O4 NPs stabilized emulsion after 3 days. Reproduced with Permission [32]
Phase separation
Phase separation is a result of the disparity in the densities of emulsion droplets and the continuous phase. The difference in droplet density tends to cause the migration of droplets up or down vertically through the continuous phase. Droplets with a lower density tend to form a layer of emulsion droplets above the continuous phase of the emulsion. This phenomenon is known as creaming. Conversely, if the droplets are denser, they form a layer at the bottom of the emulsion. This phenomenon is known as sedimentation (Scheme 5). Generally, o/w emulsions undergo creaming due to the lower density of oil relative to water, while w/o emulsions tend to sediment. Creaming or sedimentation in emulsions can easily be assessed by visual inspection and appropriate spectroscopic measurements. The rate of emulsion creaming or sedimentation can be estimated by measuring the volume fraction of the residual emulsion and/or the oil/water phase as a function of time, using appropriate techniques [7,11,12].
Wettability and contact angle
The wettability of particles used as stabilizers in Pickering emulsions is a significant factor that influences the effectiveness of particles in stabilizing emulsions. This property is usually assessed by measuring the three-phase contact angle at the o/w interface. Particles with a contact angle less than 90o are considered hydrophilic and suitable for the stabilization of o/w emulsions. On the other hand, particles with contact angle greater than 90o are hydrophobic and are generally most effective for the stabilization of w/o emulsions, including emulsions containing highly polar oils. The contact angle can be measured using a variety of methods such as the classical drop-shape method, Washburn capillary rise (WCR) method, sessile drop method, Wilhelmy plate method, microcalorimetry, atomic force microscopy (AFM) and the gel trapping technique (Figure 13) (GTT) [14,44,45].
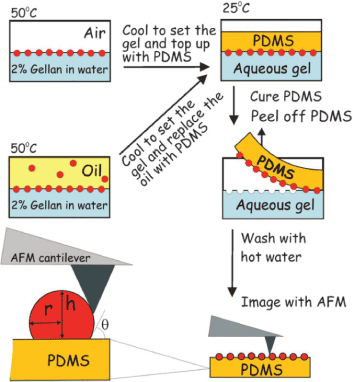
Figure 13: Schematic presentation of determination of the contact angle of NPs via a combination of the gel trapping technique (GTT) and atomic force microscopy (AFM). Reproduced with Permission [45]
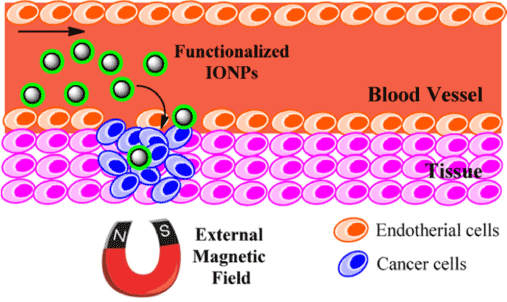
Figure 14: The application of magnetic NPs in controlled drug delivery. Reproduced with Permission [55].
Applications of Pickering emulsions
The stability of Pickering emulsions has contributed to the use of such systems in diverse applications involving colloidal phases. Various industrial applications include biomedical devices and controlled drug delivery. Drug delivery refers to the administration of a pharmaceutical in humans or animals with the aim of achieving a healing effect. The drug may be administered via various routes which include oral, nasal, topical and pulmonary, etc. [46]The properties of Pickering emulsions that justify their prominence in such applications relates to the resulting emulsion stabilization. Pickering emulsions are stabilized by the tendency of such particles to adsorb strongly at the oil/water interface, as evidenced by the formation of dense coatings on emulsion droplets. These dense coatings alter the mass transfer barrier by creating a barrier to diffusion of drug molecules. Thus, Pickering emulsion droplets serve as suitable encapsulation systems [47-49]. Moreover, the adjustable permeability of Pickering emulsions and related materials provide distinctive advantages for selected applications. The specific properties of the droplet surface such as pH and thermo-responsiveness, along with magnetic responsive behavior afford magnetic-based targeting into tissues in a different manner over conventional surfactant-based emulsions. For example, the application of drug loaded Pickering emulsions in drug delivery to skin via topical routes illustrate the significant potential of these systems as advanced drug delivery systems. Frelichowska et al.[50] reported that the delivery of a hydrophilic drug (caffeine) from w/o Pickering emulsions to the skin. The results reveal that faster delivery was observed relative to surfactant-based emulsions having similar chemical composition and viscosity. Similar results have also been reported for w/o emulsions loaded with a fluorescent probe [51] and methyl salicylate [52], respectively.
Jarzyna et. al. [53] investigated the application of iron oxide core oil/water emulsions as a multifunctional NP platform for tumor targeting and imaging. The authors reported the application of the nanoemulsions for in vitro studies, where the nanoemulsion was found to be biocompatible. Supporting evidence was found by the viability of the cells at the applied high NP dosage, in agreement with reports for other commercially available contrast agents [54]. The study also reveals that nano- emulsions were suitable for in vivo fluorescence studies as well as in vivo magnetic resonance imaging (MRI) studies. The unique properties of Fe3O4 NPs such as thermal stability and magnetic responsiveness have added benefits over other particles based on organic scaffolds. The greater thermal stability of Fe3O4 NPs provides support for their utility in stabilized Pickering emulsions for high temperature applications. In the treatment of hyperthermia, the magnetic responsiveness of such NPs enhance their application in magnetic fluids for MRI [53]. Other industrial applications of Pickering emulsions include heavy oil transport, oil recovery, emulsion polymerization, catalyst recovery and cosmetics. Pickering emulsions stabilized by NPs are also being investigated for their potential utility as alternatives or supplementary dispersants for oil-spill remediation. The tendency of the emulsifiers to self-assemble at the o/w interface is being exploited in the synthesis of microparticles, along with hollow particles [2,4,12,19,31,55]. A recent review by Dickinson [56] provides an overview on the application of Pickering emulsions in various aspects of food processing.
Future research
Although there are emerging studies on the application of cellulose biopolymers and cellulose/Fe3O4 NPs composite materials as Pickering emulsion stabilizing agents [28,57,58]. A number of such studies have employed nanocrystalline cellulose or its modified forms as the emulsifying agent due to its high S/V ratio [59]. There have been sparse reports on the application of bulk cellulose to prepare supported Fe3O4 NPs composites, especially their use as Pickering emulsion stabilizing agents. The lack of such studies may be related to the recalcitrant nature of bulk cellulose. Cellulose supported NPs in the form of composites can allow for subtle surface modification whilst enhancing NP stability. In the light of the above, future studies are suggested which may be directed, as follows: i) design anisotropic cellulose supported NPs composite with variable amphiphilic properties, aspect ratio, and polydispersity index, ii) evaluate the Pickering emulsion stabilizing efficacy of the cellulose Fe3O4 NP composites, and iii) evaluate the effects of differing levels of fibrillation (nano-, micro-, and bulk cellulose) on the emulsion stabilizing efficiency of such composites. The modified cellulose materials (hybrids) can be studied using various materials characterization techniques such as infrared (IR) spectroscopy, scanning electron microscopy (SEM) and thermal analytical methods (TGA and DSC). The emulsion stabilization efficacy of the modified cellulose will be evaluated by preparing Pickering emulsions of both polar and non-polar oils and their thermodynamic and kinetic stability can be assessed.
Conclusions
This mini-review provides an overview of the effectiveness of Fe3O4 NPs as stabilizing agents for Pickering (particle stabilized) emulsions of both polar and non-polar oils. Pickering emulsions can be prepared as o/w, w/o, and as complex (w/o/w or o/w/o) emulsions. These emulsion systems are typically prepared by wetting the stabilizing agent in the primary liquid with mechanical agitation before the addition of the secondary liquid. Among the various stabilizing agents for Pickering emulsions, Fe3O4 NPs are anticipated to be the subject of future research due to the availability, synthetic versatility, unique physicochemical properties (high surface area, ferromagnetism, etc.) and variable surface modification. The surface modification of NPs result in systems with variable hydrophile-lipophile balance which have variable adsorption affinity and stabilization of the o/w interface. Structural modification of NPs to yield variable surface functionality and textural properties will lead to further structural diversity and functional versatility as stabilizing agents for Pickering emulsions. While hydrophobic particles are suitable for stabilizing w/o emulsions, hydrophilic particles are employed as stabilizers of o/w emulsions. The modification of Fe3O4 NPs allows for the ability to tune the hydrophile-lipophile balance of NPs. In turn, this leads to stabilization of emulsions containing highly polar vs. nonpolar oils. Factors such as pH of the aqueous solution, oil/water volume ratio, polarity of the oil phase, ionic strength, nature of modification of the Fe3O4 NPs and concentration of the NPs affect the stability of Pickering emulsions. The thermodynamic and kinetic stability of Pickering emulsions can be assessed by a range of conventional spectroscopic methods such as contact angle, light scattering, and microscopy and bulk physical properties. Pickering emulsions are predicted to find increasing applications in colloidal dispersions due to the increasing need to develop sustainable and alternative stabilizers suitable for a range of applications in the production of foods, fuels, personal care products, and medicine.
Acknowledgements
The authors acknowledge the support of the Government of Saskatchewan (Ministry of Agriculture) through the Agriculture Development Fund (PROJECT#: 20110162). The University of Saskatchewan and Environment and Climate Change Canada are also gratefully acknowledged for cooperation and support.
Competing Interests
The authors declare no competing interests.
References
- Khan AY, Talegaonkar S, Iqbal Z, Ahmed FJ, Khar RK (2006) Multiple emulsions: an overview. Curr Drug Deliv 3: 429-443. [Crossref]
- Wu J, Ma GH (2016) Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 12: 4633-4648. [Crossref]
- Destribats M, Gineste S, Laurichesse E, Tanner H, Leal-Calderon F, et al. (2014) Pickering emulsions: what are the main parameters determining the emulsion type and interfacial properties? Langmuir 30: 9313-9326. [Crossref]
- Vengsarkar PS (2015) “Synthesis and Gas-Expanded Liquid (GXL) Processing of Metal and Metal Oxide Nanoparticles: Fundamentals and Application.” PhD diss., Auburn University, Accessed on August 3, 2016
- Ramsden W (1903) Separation of solids in the surface-layers of solutions and‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation). -- preliminary account. Proceedings of the Royal Society of London, 72: 156-164.
- Pickering SU (1907) CXCV—the chemistry of bordeaux mixture. J Chem Soc Trans 91: 1988-2001.
- Melle S, Lask M, Fuller GG (2005) Pickering emulsions with controllable stability. Langmuir 21: 2158-2162. [Crossref]
- Larson-Smith K, Pozzo DC (2012) Pickering emulsions stabilized by nanoparticle surfactants. Langmuir 28: 11725-11732. [Crossref]
- Lam S, Velikov KP, Velev OD (2014) Pickering stabilization of foams and emulsions with particles of biological origin. Current Opinion in Colloid & Interface Science 19:490-500.
- Binks BP, Whitby CP (2005) Nanoparticle silica-stabilised oil-in-water emulsions: Improving emulsion stability. Colloids and Surfaces A: Physicochemical and Engineering Aspects 253: 105-115.
- Zhou J, Qiao X, Binks BP, Sun K, Bai M, et al. (2011) Magnetic Pickering emulsions stabilized by Fe3O4 nanoparticles. Langmuir 27: 3308-3316. [Crossref]
- Zhou J, Wang L, Qiao X, Binks BP, Sun K (2012) Pickering emulsions stabilized by surface-modified Fe3O4 nanoparticles. J Colloid Interface Sci 367: 213-224. [Crossref]
- Ingram DR, Kotsmar C, Yoon KY, Shao S (2010) Superparamagnetic nanoclusters coated with oleic acid bilayers for stabilization of emulsions of water and oil at low concentration. J Colloid Interface Sci 351: 225-232. [Crossref]
- Lan Q, Liu C, Yang F, Liu S, Xu J, et al. (2007) Synthesis of bilayer oleic acid-coated Fe3O4 nanoparticles and their application in pH-responsive Pickering emulsions. J Colloid Interface Sci 310: 260-269. [Crossref]
- Chen T, Colver PJ, Bon SAF (2007) Organic–inorganic hybrid hollow spheres prepared from tio2-stabilized pickering emulsion polymerization. Advanced Materials 19: 2286-2289.
- Chen W, Liu X, Liu Y, Kim HI (2010) Synthesis of microcapsules with polystyrene/zno hybrid shell by pickering emulsion polymerization. Colloid and Polymer Science 288: 1393-1399.
- Xiao J, Li C, Huang Q (2015) Kafirin nanoparticle-stabilized pickering emulsions as oral delivery vehicles: Physicochemical stability and in vitro digestion profile. J Agric Food Chem 63: 10263-10270. [Crossref]
- Brown P, Bushmelev A, Butts CP, Cheng J, Eastoe J, et al. (2012) Magnetic control over liquid surface properties with responsive surfactants. Angewandte Chemie International Edition 51: 2414-2416. [Crossref]
- Orbell JD, Godhino L, Bigger SW, Nguyen TM, Ngeh LN (1997) Oil spill remediation using magnetic particles: An experiment in environmental technology. Journal of Chemical Education 74: 1446.
- Qiao X, Zhou J, Binks BP, Gong X, Sun K (2012) Magnetorheological behavior of pickering emulsions stabilized by surface-modified fe3o4 nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects 412: 20-28.
- Kaiser A, Liu T, Richtering W, Schmidt AM (2009) Magnetic capsules and pickering emulsions stabilized by core-shell particles. Langmuir 25: 7335-7341. [Crossref]
- Hu Y, Huang J, Zhang Q, Yang Y, Ma S, et al. (2015) Functional nanoparticle-decorated graphene oxide sheets as stabilizers for pickering high internal phase emulsions and graphene oxide based foam monoliths. RSC Adv 5: 103394-103402.
- Zhou J, Wang L, Qiao X, Binks BP, Sun K (2012) Pickering emulsions stabilized by surface-modified Fe3O4 nanoparticles. J Colloid Interface Sci 367: 213-224. [Crossref]
- Akimoto M, Sugibayashi K, Morimoto Y (1985) Application of magnetic emulsions for sustained release and targeting of drugs in cancer chemotherapy. Journal of Controlled Release 1: 205-215.
- Ahmed N, Jaafar-Maalej C, Eissa MM, Fessi H, Elaissari A (2013) New oil-in-water magnetic emulsion as contrast agent for invivo magnetic resonance imaging (MRI). Journal of Biomedical Nanotechnology 9: 1579-1585.
- Veyret R, Pichot TD, Elaissari A (2005) Functionalized magnetic emulsion for genomic applications. Current Organic Chemistry 9: 1099-1106.
- Cai X, Yang F, Gu N (2012) Applications of magnetic microbubbles for theranostics. Theranostics 2: 103-112. [Crossref]
- Berton-Carabin CC, Schroën K (2015) Pickering emulsions for food applications: background, trends, and challenges. Annu Rev Food Sci Technol 6: 263-297. [Crossref]
- Chevalier Y, Bolzinger MA (2013) Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids and Surfaces A: Physicochemical and Engineering Aspects 439: 23-34.
- Sacanna S, Kegel WK, Philipse AP (2007) Thermodynamically stable pickering emulsions. Phys Rev Lett 98: 158301. [Crossref]
- 31.Tang J, Quinlan PJ, Tam KC (2015) Stimuli-responsive Pickering emulsions: recent advances and potential applications. Soft Matter 11: 3512-3529. [Crossref]
- Lin Z, Zhang Z, Li Y, Deng Y (2016) Magnetic nano-fe3o4 stabilized pickering emulsion liquid membrane for selective extraction and separation. Chemical Engineering Journal 288: 305-311.
- Duan H, Wang D, Sobal NS, Giersig M, Kurth DG, et al. (2005) Magnetic colloidosomes
- derived from nanoparticle interfacial self-assembly. Nano Lett 5: 949-952. [Crossref]
- Lin KYA, Yang H, Petit C, Lee WD (2015) Magnetically controllable pickering emulsion prepared by a reduced graphene oxide-iron oxide composite. J Colloid Interface Sci 438: 296-305. [Crossref]
- Lin Z, Yu D, Li Y (2014) Study on the magnetic odsa-in-water pickering emulsion stabilized by Fe3O4 nanoparticle. Colloid and Polymer Science 293: 125-134.
- Liu H, Wang C, Gao Q, Chen J, Liu X, et al. (2009) One-pot fabrication of magnetic nanocomposite microcapsules. Materials Letters 63: 884-886.
- Zou S, Wang C, Gao Q, Tong Z (2013) Surfactant-free multiple pickering emulsions stabilized by combining hydrophobic and hydrophilic nanoparticles. Journal of Dispersion Science and Technology 34: 173-181.
- Keungputpong N, Matchariyakul N, Tiptipakorn S (2014) A study of the effect of starch in polymer blends of carboxymethyl cellulose and chitosan. Advanced Materials Research 2014: 989-994.
- Ling D, Lee N, Hyeon T (2015) Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Acc Chem Res 48: 1276-1285. [Crossref]
- Józefczak A, Wlazlo R (2015) Ultrasonic studies of emulsion stability in the presence of magnetic nanoparticles. Advances in Condensed Matter Physics 2015: 1-9.
- Wu W, He Q, Jiang C (2008) Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Research Letters 3: 397-415.
- Binks BP, Rodrigues JA (2003) Types of phase inversion of silica particle stabilized emulsions containing triglyceride oil. Langmuir 19: 4905-4912.
- Binks BP, Lumsdon SO (2001) Pickering emulsions stabilized by monodisperse latex particles: Effects of particle size. Langmuir 17: 4540-4547.
- Alghunaim A, Kirdponpattara S, Newby B (2016) Techniques for determining contact angle and wettability of powders. Powder Technology 287: 201-215.
- Arnaudov LN, Cayre OJ, Cohen Stuart MA, Stoyanov SD, Paunov VN (2010) Measuring the three-phase contact angle of nanoparticles at fluid interfaces. Phys Chem Chem Phys 12: 328-331. [Crossref]
- Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, et al. (2012) Drug delivery systems: An updated review. Int J Pharm Investig 2: 2-11. [Crossref]
- Tan A, Simovic S, Davey AK, Rades T, Prestidge CA (2009) Silica-lipid hybrid (SLH) microcapsules: a novel oral delivery system for poorly soluble drugs. J Control Release 134: 62-70. [Crossref]
- Prestidge CA, Simovic S (2006) Nanoparticle encapsulation of emulsion droplets. Int J Pharm 324: 92-100. [Crossref]
- Simovic S, Prestidge CA (2007) Nanoparticle layers controlling drug release from emulsions. Eur J Pharm Biopharm 67: 39-47. [Crossref]
- Frelichowska J, Bolzinger MA, Valour JP, Mouaziz H, Pelletier J, et al. (2009) Pickering w/o emulsions: drug release and topical delivery. Int J Pharm 368: 7-15. [Crossref]
- Eskandar NG, Simovic S, Prestidge CA (2010) Mechanistic insight into the dermal delivery from nanoparticle-coated submicron O/W emulsions. J Pharm Sci 99: 890-904. [Crossref]
- Marku D, Wahlgren M, Rayner M, Sjöö M, Timgren A (2012) Characterization of starch Pickering emulsions for potential applications in topical formulations. Int J Pharm 428: 1-7. [Crossref]
- Jarzyna PA, Skajaa T, Gianella A, Cormode DP, Samber DD, et al. (2009) Iron oxide core oil-in-water emulsions as a multifunctional nanoparticle platform for tumor targeting and imaging. Biomaterials 30: 6947-6954. [Crossref]
- Wang Y, Ng YW, Chen Y, Shuter B, Yi J, et al. (2008) Formulation of superparamagnetic iron oxides by nanoparticles of biodegradable polymers for magnetic resonance imaging. Adv Funct Mater 18: 308-318.
- Wu W, Wu Z, Yu T, Jiang C, Kim WS (2016) Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications.? Sci Tech Adv Mater 16: 023501.
- Dickinson E (2015) Colloids in food: ingredients, structure, and stability. Annu Rev Food Sci Technol 6: 211-233. [Crossref]
- Zoppe JO, Venditti RA, Rojas OJ (2012) Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes. J Colloid Interf Sci 369: 202-209. [Crossref]
- Nypelö T, Rodriguez-Abreu C, Kolen’ko YV, Rivas J, Rojas OJ (2014) Microbeads and hollow microcapsules obtained by self-assembly of pickering magneto-responsive cellulose nanocrystals. ACS Applied Materials & Interfaces 6: 16851-16858.
- Udoetok IA, Wilson LD, Headley JV (2016) Quaternized Cellulose Hydrogels as Sorbent Materials and Pickering Emulsion Stabilizing Agents. Materials 9: 645-661.



















