Objective: Systemic sclerosis (SSc) is primarily characterized by autoimmunity, microangiopathy, and tissue fibrosis. Hypoxia, a powerful stimulator of vascular endothelial growth factor (VEGF), may be responsible for excessive expression of chronic VEGF in SSc. The oxygen-regulated α-subunit of hypoxia-inducible transcription factor-1 (HIF-1α) plays an important role in transcriptional regulation of VEGF. The protein phosphatase and tensin homolog (PTEN) is responsible for dephosphorylation of proteins and thereby promotes tissue repair.
The present study aimed to examine the expressions of HIF-1α, PTEN, and VEGF in patients with scleroderma in an attempt to estimate prognosis and guide therapeutic decisions. We therefore studied the expressions of HIF-1 α, VEGF, and PTEN in skin samples exhibiting the features of scleroderma.
Methods: Skin biopsies of patients were obtained and samples were stained with primary antibodies following PTEN, VEGF and HIF-1α, immunohistochemically.
Results: The SSc and the control group were compared with respect to staining of epidermal keratinocytes, fibroblasts, and the endothelial cells for the antibodies. SSc patients displayed a reduced VEGF expression in endothelial cells. The lesion group showed a significantly lower staining of epidermal cells and endothelial cells for PTEN. SSc group was also characterized by a significantly weaker HIF-1 α expression compared to the control group.
Conclusion: In scleroderma skin samples, manipulated expression of these molecules at varying stages of the disease may affect the prognosis of scleroderma.
HIF-1α, PTEN, Scleroderma, VEGF
Scleroderma or systemic sclerosis is a complex connective tissue disease which is characterized by vasculopathy, autoimmunity and progressive fibrosis in the skin and visceral organs [1-3]. It includes diffuse and limited subgroups. These groups are separated from each other by skin involvement [4]. Despite some characteristics distinguishing the two types from each other, they share the common feature of fibrosis involving excessive extracellular matrix (ECM) production that impairs usual tissue characteristics to cause organ failure late in the disease course [5]. Although a large body of novel scientific knowledge has been acquired about the molecular mechanisms of pathophysiology of SSc [6-8], we are far from stopping or even slowing down the ongoing fibrotic process characterizing the disease [9-11].
In this immunohistochemical study, we aimed to find new markers to predict prognosis and guide therapy.
Skin biopsies of 43 patients were obtained from the specimen archives of Mersin University Medical School Pathology Department. Twenty seven patients were compatible with scleroderma in histologically and clinically. Sixteen cases with intact surgical margins of malignant melanomas were used as a control group.
Immunohistochemical study was performed by avidine-biotin-peroxidase complex method using 4 micrometer thick sections embedded in paraffin. Gradual ethanol solutions were used for deparaphinization and dehydration. The samples were treated with 0.3% hydrogen peroxidase and methanol for 20 minutes to block endogenous peroxidase activity. Following three rinses in phosphate-buffered saline (PBS), the samples were processed in a 0.01 M citrate buffer (pH 6.0), stored in microwave for 20 minutes, and allowed to cool down at room temperature. The sections were incubated with the primary antibodies following PTEN (Santa Cruze, Sc- 133242, 1/100 dilution, Texas, U.S.A), VEGF (Santa Cruze, sc-152, 1/100 dilution, Texas, U.S.A), and HIF-1α (Santa Cruze, Sc-10790, 1/50 dilution, Texas, U.S.A) at 4°C followed by the secondary antibody. AEC-substrate chromogen (Dako, k3464, Denmark) was used to visualize the binding sites of peroxidase activity. The sections were counterstained with hematoxylin. Light microscopy (Olympus, Bx53, Tokyo, Japan) was used to detect immunostaining by two pathologists who were blind to the clinicopathological properties. Each immunohistochemistry staining was scored by the modified method of Distler, et al. [12]. The immunostaining samples were graded in varied grades ranging from no or weak expression to moderate-to-severe expression by the cells including keratinocytes, fibroblast and endothelial cells (cytoplasmic staining for PTEN and VEGF, nuclear staining for HIF-1α) on 5 high power fields (HPF). Accordingly, 1(+) staining equaled no or weak staining of a few cells (≤ 25%) and 2(+) staining equaled moderate or intense staining of the cells (25%).
Statistical analysis
Descriptive statistics were used to express study data. Inter-group analyses were performed using ShapiroWilk, Mann Whitney U, and Kruskal Wallis tests. A p value less than 0.05 was considered statistically significant. MedCalc ®v.10.3.0 software (Medcalcturkey, Belgium) was used for all statistical analyses.
Demographic, clinical and pathological findings
The histopathological properties of skin biopsy samples from patients with SSc mainly included perivascular inflammatory infiltrates, perivascular edema, and collagen accumulation of variable extent in papillary and reticular layers of dermis (Figure 1A). In addition, dermal papillary flattening, prominently reduced microvascular density, and severe fibrotic changes manifested by collagen bundles that were densely stored and showed irregular distribution were evident (Figure 1B). The SSc group, as compared to the control group, was significantly younger than the control group at the time of biopsy (42.63 ± 18.79 vs. 60.63 ± 12.53 years, p=0.002), and it contained significantly more females than males (81.5% vs. 31.3, %, p<0.001). Nevertheless, the lesion thickness did not differ significantly between the lesion and the control group (Table 1).
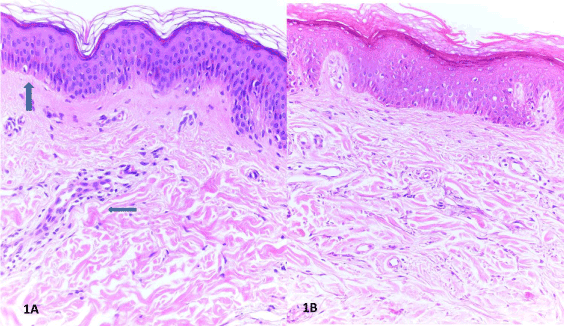
Figure 1. A. Histological features of Ssc including fibrosis of papillary and reticular dermis B. Ssc represents flattening of dermal papilla and edema around the microvessels (H-E,x100). C. Normal skin sample (H-E,x100).
Table 1. Demographic data of the patient and control groups.
SD / n / % / p |
SSc |
Control |
p |
Age |
42.63±18.79 |
60.63±12.53 |
0.002 |
Sex |
Male |
Female |
Male |
Female |
0.001 |
n |
% |
n |
% |
n |
% |
n |
% |
5 |
(18.5) |
22 |
(81.5) |
11 |
(68.8) |
5 |
(31.3) |
Thickness |
3cm (SD:2-5cm.) |
5.50cm (SD:3-8cm) |
0.092 |
SSc: Scleroderma, SD: standart deviation, n:number of cases, p: significance, %: percentage
Immunohistochemical findings
The SSc and the control group were compared with respect to staining of epidermal keratinocytes, fibroblasts, and the endothelial cells for PTEN, HIF-1α, and VEGF. The control group exhibited a variable PTEN expression in dermal vascular endothelial cells, fibroblasts, and epidermal keratinocytes. The lesion group showed a significantly lower staining of epidermal cells and endothelial cells for PTEN than the control group, respectively (81.5% vs. 18.8% number/5 HPF and 74.1% vs. 18.8% number/5 HPF), (for both p<0.0001). Both epidermal and microvascular expression were either undetectable or absent. This contrasted the skin samples from the patients with SSc, which showed significantly increased PTEN expression in fibroblasts of dermis and had a significantly higher immunopositivity (%77.8 vs. 18.8% number/5 HPF) (p<0.0001) (Figure 2A–C). The patient group had a significantly lower VEGF staining of endothelial cells (74.1% vs. 6.3% number/5 HPF) (p<0.001) than the control group. SSc patients displayed a reduced VEGF expression in endothelial cells (Figure 3A–C). The both groups, however, did not significantly differ from each other with regard to epidermal cell and fibroblast staining for VEGF. The both groups exhibited a weak and heterogeneous immunostaining in keratinocytes and fibroblasts. The SSc group was also characterized by a significantly weaker HIF-1 α expression compared to the control group (77.8% vs. 18.8% number/5 HPF), (p<0.001). The control subjects had a more prominent HIF-1α immunostaining in keratinocytes albeit to a lesser extent in some fibroblasts and endothelial cells. Dermal microvessels and fibroblasts displayed a weak and similar HIF-1α expression in both groups. The both groups were not significant with regard to HIF-1α expression (Figure 4A-C) (Table 2).
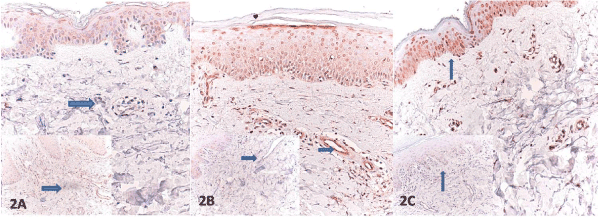
Figure 2. A. Decreased expression of PTEN of the fibroblasts (arrow) (inset:increased expression of PTEN in control) B. Increased PTEN expression of endothelial cells (arrowhead) )(inset: decreased expression of PTEN in endothelial cells in control) and C. Increased PTEN expression of keratinocytes (arrowhead) )(inset: decreased expression of PTEN in keratinocytes in control) (PTEN, x100).
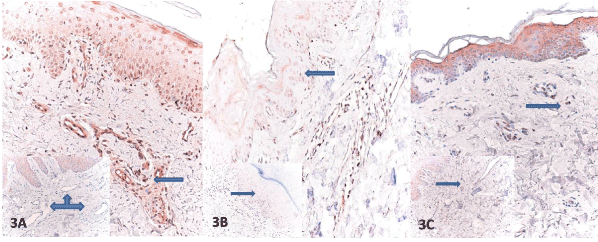
Figure 3. A. Endothelial cells showing reduced expression of VEGF (arrow) )(inset: increased expression of VEGF in endothelial cells in control) B. Moderate staining of VEGF in keratinocytes (arrow) in Ssc and control group (inset) C. Similar staining of VEGF in fibroblasts in Ssc (arrowhead) and in control (inset) (VEGF, x100).
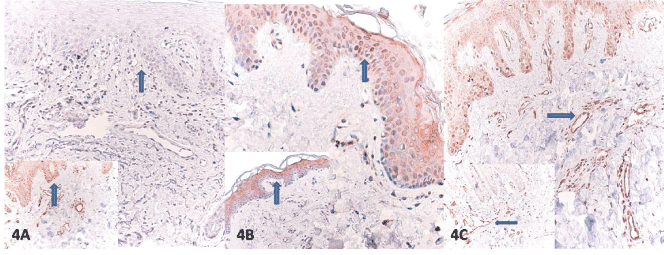
Figure 4. A. Weak nuclear HIF-1α staining in keratinocytes (arrow) in Ssc in contrast to strong staining in control group (inset) B. Strong nuclear HIF-1α staining only rarely occurs in normal endothelial cells in Ssc and control group(inset). C. Similar exhibition of HIF-1α in Ssc and control group (inset) in fibroblasts (arrow) (HIF-1α, X100).
Table 2. Immunohistochemical findings of the patient and control groups.
Immunohistochemistery
(n, %, p) |
Location |
Score |
Control group |
Scleroderma group |
p |
n |
% |
n |
% |
PTEN
|
Epidermis
|
1 |
3 |
18.8 |
22 |
81.5 |
<0.001 |
2 |
13 |
81.3 |
5 |
18.5 |
Fibroblast
|
1 |
13 |
81.3 |
6 |
22.2 |
<0.001 |
2 |
3 |
18.8 |
21 |
77.8 |
Endothelium |
1 |
3 |
18.8 |
20 |
74.1 |
<0.001 |
2 |
13 |
81.3 |
7 |
25.9 |
VEGF |
Epidermis
|
1 |
15 |
93.8 |
20 |
74.1 |
0.087 |
2 |
1 |
6.3 |
7 |
25.9 |
Fibroblast
|
1 |
14 |
87.5 |
21 |
77.8 |
0.418 |
2 |
2 |
12.5 |
6 |
22.2 |
Endothelium |
1 |
1 |
6.3 |
20 |
74.1 |
<0.001 |
2 |
15 |
93.8 |
7 |
25.9 |
HIF-1α |
Epidermis
|
1 |
3 |
18.8 |
21 |
77.8 |
<0.001 |
2 |
13 |
81.3 |
6 |
22.2 |
Fibroblast
|
1 |
15 |
93.8 |
24 |
88.9 |
0.586 |
2 |
1 |
6.3 |
3 |
11.1 |
Endothelium |
1 |
15 |
93.8 |
24 |
88.9 |
0.586 |
2 |
1 |
6.3 |
3 |
11.1 |
2021 Copyright OAT. All rights reserv
SSc is primarily characterized by autoimmunity, microangiopathy, and tissue fibrosis. A reduced capillary density results in decreased blood flow and tissue oxygenation. Initially, ECM accumulation around blood vessels leads to reduced transport of oxygen and nutrition to tissues. Severe hypoxia in turn may cause the disease to progress by stimulating the production and release of ECM proteins from fibroblasts in response to increased levels of transforming growth factor-β. The stimulation of increased production and release of ECM proteins is mediated by hypoxia-inducible factor-1α-dependent and -independent pathways. It is also possible that vascular disease be induced or worsened by hypoxia-induced VEGF receptor signaling since hypoxia represents a strong inducer of VEGF production. This leads to over-expression of VEGF in SSc, which causes to exert some unwanted effects on angiogenesis owing to formation of a non-uniform and chaotic vascular distribution and reduces blood flow. These effects, by increasing vascular disease and augmenting tissue fibrosis, jointly play a role in the pathogenesis of SSc [13]. SSc is characterized by progressive dilatation of and leakage from the skin capillaries, which in time coalesce to form extensive avascular areas and ultimate formation of digital ulcers. VEGF is consistently increased throughout the disease course and had negligible effects on neovascularization. The central event in transcriptional regulation of VEGF is the HIF-1α production regulated by oxygen [14]. VEGF levels have been found increased in early-stage SSc and this finding has been linked to pulmonary fibrosis and abnormal pulmonary function. A high level of VEGF has also been correlated to a shorter disease duration and rapidly progressive diffuse cutaneous SSc. However, some studies have reported contradictory results [15]. Our results showed a decreased VEGF expression in endothelial cells obtained from skin samples of patients with scleroderma. Scleroderma is characterized by vascular endothelial injury that is responsible for pathological alterations of vascular tissue, resulting in adverse events in physiology of many organ systems and culminating into chronic tissue ischemia. The tissue response to the hypoxia includes complex cellular and molecular mechanisms aimed to salvage endothelial functions and tissue perfusion. Both progressive capillary loss and progressive arteriolar vascular modeling lead to reduction of blood supply with associated severe and chronic hypoxia. Our results indicating reduced VEGF expression in endothelial cells may be related to loss of skin capillaries [16].
One study reported that SSc patients had lower HIF-1α levels in skin compared to healthy controls [12]. Skin samples from SSc patients were not characterized by increased HIF-1α expression as detected by immunohistochemistry. While healthy controls displayed a moderate-to-high HIF-1α staining in epidermis, SSc patients showed HIF-1α expression that was confined to single keratinocytes. Both SSc patients and healthy individuals had no detectable HIF-1α protein levels. Furthermore, SSc patients had no HIF1α expression pattern that was correlated to upregulated VEGF levels, which are among the main transcriptional targets of HIF-1α.12
In SSc, decreased HIF-1α levels in fibrotic skin may be a result of PHD-dependent HIF-1α negative feedback loops occurring in chronic hypoxic conditions [13]. Our results also showed a decreased HIF-1α expression in epidermal keratinocytes. Hypoxia-inducible factor-1 (HIF-1) mediates cellular responses to hypoxia. HIF-1 expression may be reduced in response to hypoxia in scleroderma. The fate of stem cells, i.e differentiation or proliferation, is highly dependent on oxygen content that regulates expression of various genes via hypoxia-inducible factor-1 (HIF-1). Scleroderma may benefit from hyperbaric oxygen therapy [17]. Marcus, et al. demonstrated that hyperbaric oxygen therapy well succeeded in treatment of ischemic scleroderma lesions. Hyperbaric oxygen can also be useful for treating Raynaud’s phenomenon in scleroderma [18]. It may boost hypoxia-inducible factor-1 expression, which may be a useful marker for scleroderma activation in future. In many diseases, fibrosis is a common process with no known effective therapy, which ultimately causes organ failure and results in a high mortality rate. The common elements of fibrotic process in any human tissue are dysregulated repair and excessive scar formation. The PTEN has a dephosphorylating action on proteins, and this effect is responsible for tissue repair and, possibly, pathological fibrosis [19]. Overly active and/or continuous tissue repair underlies fibrosis. A defect in the production and/or activity of proteins that are responsible for suppressing the fibrosing activity may be responsible for this occurrence. PTEN may be one of those proteins [20]. However, it is not entirely clear if a defective PTEN/Akt pathway is responsible for chronic fibrosis of SSc. It is equally controversial that defective PTEN expression is enough for emergence of a fibrotic phenotype [19]. We found that PTEN expression was absent in epidermal keratinocytes and endothelial cells despite an increase in fibroblasts in SSc patients. It has been showed by some functional studies that PTEN is critically important in suppressing skin cancer and loss of its activity may lead to skin tumor development by a variety of mechanisms including mutation, deletion, or reduced expression [21]. Studies in humans and mice have demonstrated that PTEN is of vital importance for skin homeostasis and it is critically important for suppression and thus prevention of skin tumors. It has been suggested that PTEN exerts its suppressing effects on skin tumors by antagonizing PI3K/AKT pathways. Proliferation and balance of epidermal cells, including keratinocytes and melanocytes, are critically dependent on a normal PTEN function in order to suppress their transformation. A heightened PTEN expression in scleroderma may actually reduce fibroblast spread and emigration. Moreover, PTEN activity is suppressed by ultraviolet (UV) radiation, suggesting that skin carcinogenesis induced by UV radiation is highly dependent on loss of PTEN activity. It is also interesting that UV phototherapy benefits patients with scleroderma [22]. It may be advocated that this beneficial effect is a result of inhibition of PTEN expression.
A lower VEGF expression in endothelial cells, a lower HIF-1α expression in keratinocytes, and a variable PTEN expression in scleroderma skin samples (higher expression in fibroblasts and lower in endothelial cells and keratinocytes) were demonstrated by our study. Such a pattern of expression is of particular importance since they have a critical role in emergence of scleroderma. Results of other studies using some different methods for scleroderma therapy may worth being compared with our results as they would offer a unique insight into their ability to alter the expression of these molecules and initiate certain molecular pathways that lead to scleroderma. Furthermore, pathophysiology and cellular events in development of scleroderma may be clarified by a more detailed analysis of these mediators. Further studies on this topic will shed light on the question if manipulated expression of these molecules at varying stages of the disease is a direct effect of scleroderma or they result from the altered number of skin cells responsible from production of these molecules. We hope that our study will introduce some novel fibrosis markers and their inhibitors that may be used in treatment of scleroderma.
- Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, et al. (2010) The immunology of fibrosis: innate and adaptive responses. Trends Immunol 31: 110-119. [Crossref]
- Rosenbloom J, Castro SV, Jimenez SA (2010) Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med 152: 159-166. [Crossref]
- Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M (2010) Mechanisms in the loss of capillaries in systemic sclerosis: angiogenesis versus vasculogenesis. J Cell Mol Med 14: 1241-1254. [Crossref]
- Hachulla E, Launay D (2011) Diagnosis and classification of systemic sclerosis. Clin Rev Allergy Immunol 40: 78-83. [Crossref]
- Varga J, Pasche B (2009) Transforming growth factor ß as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 5: 200-206. [Crossref]
- Rosenbloom J, Castro SV, Jimenez SA (2010) Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med 152: 159-166. [Crossref]
- Szodoray P, Kiss E (2010) Progressive systemic sclerosis - from the molecular background to innovative therapies. Front Biosci (Elite Ed) 2: 521-525. [Crossref]
- Smith G, Chan E (2010) Molecular pathogenesis of skin fibrosis: insight from animal models. Curr Rheumatol Rep 12: 26-33. [Crossref]
- Postlethwaite AE, Harris LJ, Raza SH, Kodura S, Akhigbe T (2010) Pharmacotherapy of systemic sclerosis. Expert Opin Pharmacother 11: 789-806. [Crossref]
- Asano Y (2010) Future treatments in systemic sclerosis. J Dermatol 37: 54-70. [Crossref]
- Man XY, Finnson KW, Baron M, Philip A (2012) CD109, a TGF-b co-receptor, attenuates extracellular matrix production in scleroderma skin fibroblasts. Arthritis Res Ther 14: R144. [Crossref]
- Distler O, Distler JH, Scheid A, Acker T, Hirth A, et al. (2004) Uncontrolled Expression of Vascular Endothelial Growth Factor and Its Receptors Leads to Insufficient Skin Angiogenesis in Patients With Systemic Sclerosis. Circ Res 95: 109-116. [Crossref]
- Beyer C, Schett G, Gay S, Distler O, Distler JH (2009) Hypoxia. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res Ther 11: 220. [Crossref]
- Ioannou M, Pyrpasopoulou A, Simos G, Paraskeva E, Nikolaidou C, et al. (2013) Upregulation of VEGF expression is associated with accumulation of HIF-1α in the skin of naive scleroderma patients. Mod Rheumatol 23: 1245-1248. [Crossref]
- Castro SV, Jimenez SA (2010) Biomarkers in systemic sclerosis. Biomark Med 4: 133-147. [Crossref]
- Cipriani P, Marrelli A, Liakouli V, Di Benedetto P, Giacomelli R (2011) Cellular players in angiogenesis during the course of systemic sclerosis. Autoimmun Rev 10: 641-646. [Crossref]
- Haque N, Rahman MT, Abu Kasim NH, Alabsi AM (2013) Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Scientific World Journal 2013: 632972. [Crossref]
- Marcus M, Ilyas M, Tolaymat A (2010) Childhood scleromyositis with a negative PM/Scl antibody. Joint Bone Spine 77: 73-75. [Crossref]
- Parapuram SK, Shi-wen X, Elliott C, Welch ID, Jones H, et al. (2011) Loss of PTEN Expression by Dermal Fibroblasts Causes Skin Fibrosis. Journal of Investigative Dermatology 131: 1996–2003. [Crossref]
- Tsugawa K, Jones MK, Sugimachi K, Sarfeh IJ, Tarnawski AS (2002) Biological role of phosphatase PTEN in cancer and tissue injury healing. Front Biosci 7: e245-251. [Crossref]
- Gambichler T, Sand M, Skrygan M (2013) Loss of 5-hydroxymethylcytosine and ten-eleven translocation 2 protein expression in malignant melanoma. Melanoma Res 23: 218-220. [Crossref]
- Zhao B, Ming M, He YY (2013) Suppression of PTEN transcription by UVA. J Biochem Mol Toxicol 27: 184-191. [Crossref]




