A case demonstrates a patient with the origin of all three coronary arteries. Left anterior descending (LAD), right coronary (RCA) and circumflex (CX) arteries originated from the same right aortic sinus. This anomaly combined with an apical form of hypertrophic cardiomyopathy (HCM).
The rare case of the combination of apical HCM and rare congenital coronary anomaly depicts high diagnostic value of modern imaging modalities (first of all, CT and MRI) in difficult diagnostic situations. CT with myocardial delayed enhancement depicted an area of intramyocardial fibrosis which was in concordance with late gadolinium-enhanced cardiac MRI.
heart, myocardium, hypertrophy, hypertrophic cardiomyopathy, MRI, fibrosis, ischemia, scar
Congenital anomalies of coronary arteries are relatively frequently found in general population (their incidence reaches 0.2-0.9%) [1,2]. Most of them are benign and do not require interventional or surgical treatment, but in some cases them can result in sudden death or myocardial infarction [3]. Coronary CT-angiography (CTA) or MR-angiography are effective imaging modalities for non-invasive diagnosis of this pathology [4,5]. Some variants of coronary anomalies are rarer than other. Origin of all three coronary arteries is one of the less frequent variants of aberrant coronary anatomy. Congenital coronary anomalies could be found in patients with different types of congenital cardiac or acquired diseases. Hypertrophic cardiomyopathy (HCM) is the most common of primary cardiomyopathies [6,7]. There are several forms of HCM and the apical form of HCM is one of them. This type of disease is well-known, but it occurs rarer than «classical» form of HCM with asymmetrical hypertrophy of interventricular septum (IVS). In clinical settings diagnosis of apical HCM could be difficult due to many masks of this disease. Echocardiography, cardiac MRI and (to some extent), cardiac CTA play an important role in HCM imaging and risk stratification of patients [8-10]. We are presenting a case of rare congenital coronary anomaly combined with the apical form of HCM in patient with initial suspicion to myocardial ischemia and coronary artery disease (CAD).
Male patient, 44 years old, was admitted to hospital with complaints to episodes of dyspnea, palpitations and chest pain not typical for angina pectoris. These complaints appeared approximately 4 years ago. The patient had no records of any familial or genetic cardiac diseases.
Physical examination revealed some tachycardia (100-110 bpm), mild systolic cardiac murmur, slightly increased diastolic BP (140/90 Hg) and no other abnormalities. Results of blood tests (including levels of cardiac troponin) were in normal ranges.
ECG (Figure 1) revealed sinus tachycardia (heart rate 100 bpm), prominent signs of left ventricular (LV) , myocardial hypertrophy with inverted T waves in I,II, aVL and V2-6 leads with mild ST-segment depression.
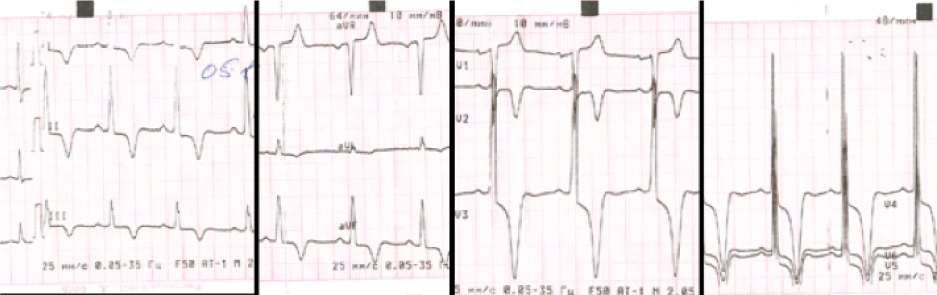
Figure 1. ECG at rest. Marked signs of LV hypertrophy and repolarization disorders are seen.
Echocardiography demonstrated that heart chambers were not enlarged and LV EF was normal (71%). The myocardial thickness of intraventricular septum (IVS and LV postero-lateral wall in the basal segments) was mildly increased to 11-13 mm. LV apical hypertrophy was suspected. There was mild mitral and tricuspid valvular regurgitation. No signs of pulmonary hypertension were found.
Holter ECG monitoring (24 h) discovered tachycardia-related episodes of ST-segment depression up to -1.5 mm, no arrhythmias or conduction anomalies.
Stress-test (veloergometry) revealed ST-depression (-1.6 mm) during maximal workload (100 watts) with the short episode of dyspnea. The test was reported as inconclusive because of the presence of marked repolarization changes in rest ECG caused by LV myocardial hypertrophy (see above).
In order to exclude the presence of CAD, the patient was referred to coronary CTA and cardiac MRI.
Coronary and cardiac CT was performed with single-tube 64-row scanner (Discovery 750, GE Healthcare, USA) with dual-energy option (Gemstone Spectral Imaging (GSI). Coronary CTA was done in low-dose mode (tube current 100 kVp, automatic regulation of mA, slice thickness 0.625 mm, tube rotation 350 ms) with prospective ECG gating. CTA was done with 60– 80 ml (based on patients weight) of iopamidol 370 mg I/ml (Scanlux, Sanochemia, Austria), injected into an antecubital vein through a 18–20 gauge IV cannula using a power injector at a rate of 4-5 ml/s plus 40 mL of saline flush after the contrast injection. Bolus tracking was used to define the start of scanning. The delayed phase for assessment of myocardial delayed enhancement (MDE) was initiated in GSI mode 8 min after the first CTA using additional drip infusion of 50 ml of the same contrast media with rate of 0.1 ml/s approx. The study was done with fast alternated 80 kVp and 140 kVp tube current switching and a constant tube current of 600 mAs.
The multiplanar reformations and vessel maps were created for assessment of coronary vessels and heart chambers. GSI datasets were decomposed into water and iodine attenuation maps and monoenergetic images with different keV settings.
Cardiac MRI ((1.5T scanner, Avanto, Siemens, Germany) with the standard black-blood and cine-sequences was done during breathless along long- and short-axes of the LV. Late myocardial gadolinium enhancement (LGE) was performed after 15 min of IV injection of gadodiaminde (Omniscan, GE Healthcare, USA) in dose 0.3 ml/kg body weight.
Coronary and cardiac CT discovered rare congenital anomaly or coronary artery tree. All three major coronary arteries (left anterior descending (LAD), right coronary (RCA) and circumflex (CX) arteries) originated from the right Valsalva sinus (Figure 2). LAD and RCA had common ostium, CX had a separate one. LAD had an anterior course in front of pulmonary artery trunk and ascending aorta (benign type), CX had the retroaortic course (a benign type too). No signs of coronary atherosclerosis were found. CTA confirmed the presence of apical myocardial hypertrophy compatible with the diagnosis of apical HCM (Figure 3). Thickness of LV myocardium in the apical segment was 23 mm. The presence of coronary anomaly and lack of coronary stenosis were confirmed with help of cardiac catheterization (Figure 4) but origins of coronary vessels from the right coronary sinus were better seen with CTA. MDE CT revealed strong enhancement of hypertrophied apical segments of LV myocardium indicative of intramyocardial fibrosis in HCM (Figure 5).
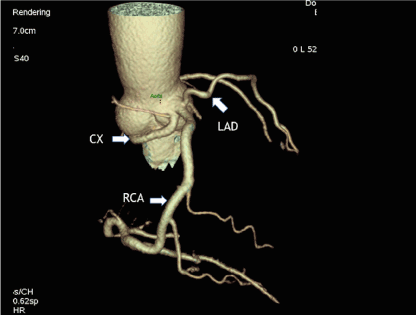
Figure 2. Coronary CTA, VRT reconstruction of coronary arteries and ascending aorta. Anomalous origin of LAD and CX from the right aortic sinus (arrow). Normal origin and position of dominant RCA (arrow).
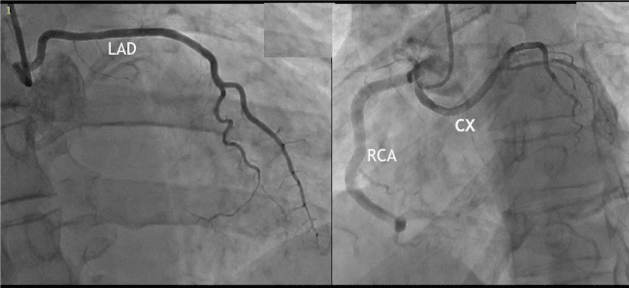
Figure 3. Coronary catheterization confirms results of coronary CTA. Anomalous origin of LAD and CX from the right aortic sinus.
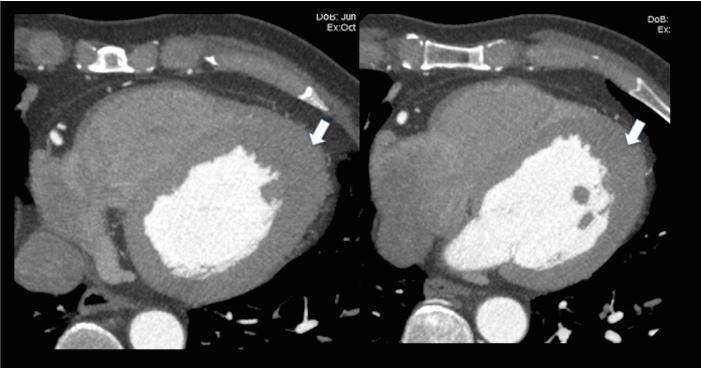
Figure 4. Coronary CTA, axial slices. Apical hypertrophy of LV myocardium is shown with arrows.
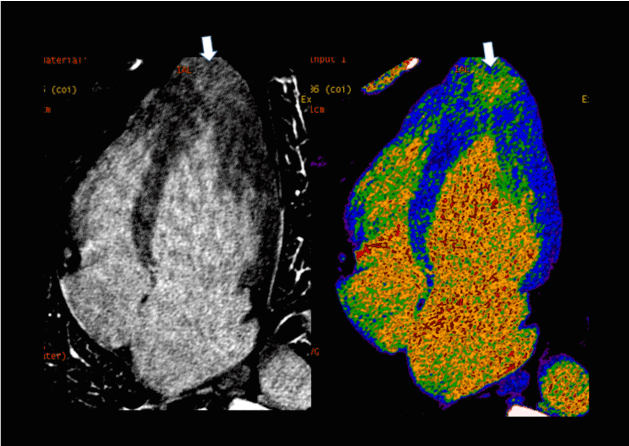
Figure 5. MDE CT, long-axis LV reformations. Left side - monoenergetic 70 keV images, right side - c color iodine map. Areas of intramyocardial delayed accumulation of contrast media are indicated with arrows.
Cardiac MRI with late gadolinium enhancement (LGE) (1.5T scanner, Avanto, Siemens, Germany) was done in order to confirm results of MDE CT. MR images revealed a typical for apical HCM systolic configuration of LV cavity («ace of spades») with obliteration of apical part of LV cavity (Figure 6). LV EF was 78%. Results of LGE-MRI were in full concordance with MDE CT. Marked intramyocardial enhancement of apical myocardium was seen on LGE images (Figure 7).
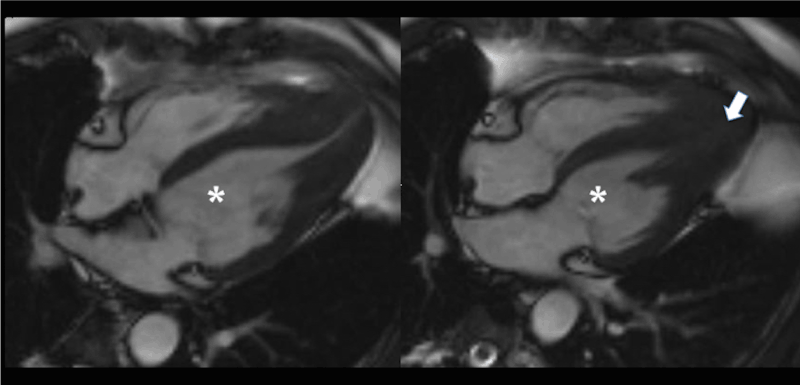
Figure 6. Cardiac cine MRI. Long-axis views. Left side - diastole, right side - systole. LV cavity is marked with the asterisk. Obliteration of LV apical cavity in systole is marked with an arrow.
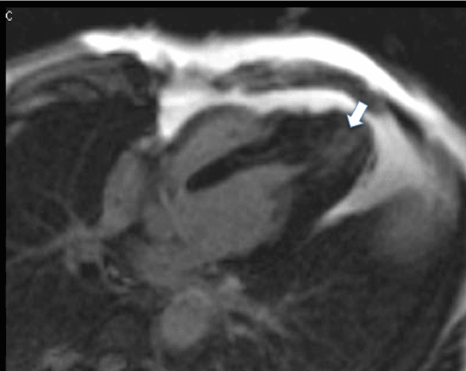
Figure 7. LGE-MRI. An area of intramyocardial fibrosis in the LV apex is marked with the arrow (concordance with results of MDE CT).
According to results of cardiac imaging modalities and other tests, the patient was attributed to the low-risk group concerning probability of sudden cardiac death. Implantation of cardioverter-defibrillator was not indicated [11]. ST-depression during the stress test and Holter ECG was estimated in this case as a false-positive result related to prominent LV hypertrophy at they were not indicative of CAD. Long-term treatment with cardioselective beta-blockers and control of BP and HR together with follow-up by the cardiologist were selected for further patient management.
2021 Copyright OAT. All rights reserv
This case in interesting for several reasons.
Different variants of HCM (including the apical form of this pathology) and different variants of coronary tree anomalies have been extensively described in the scientific literature. It is well known that patients with HCM have high occurrence of tunneled coronary arteries (myocardial bridges). But according to our knowledge, there were no reports in the literature about a combination of apical HCM with the origin of all three coronary vessels from the right aortic sinus.
Intramyocardial fibrosis of hypertrophied myocardium in HCM patients is an important biomarker which may have a prognostic significance in this disease, especially in patients with severe arrhythmias. LGE-MRI is regarded as a reference diagnostic modality for detection of this HCM feature. But this case shows that cardiac CT in dual-energy mode could detect MDE in non-coronary diseases as well as in patients with CAD. It may be of importance in patients with contraindications to MRI (e.g. in cases of pacemakers of implantable defibrillators non-compatible with MR) or when a simultaneous assessment of coronary tree is needed. For example, in this case or diagnosis of apical HCM combination with rare form of benign coronary artery anomaly (together with detection of intramyocardial fibrosis) could had been done just with help of CTA and CT MDE, without cardiac catheterization and LGE-MRI (they were performed in this patient to verify results of CT).
Modern CT has opportunities of combined analysis of coronary arteries, heart chambers, ventricle function and myocardium (MDE). It makes CT competitive to cardiac MRI not only in CAD but also in non-coronary disease. Coronary and cardiac CT have one drawback - it is an additional radiation exposure to the patient. But it has decreased significantly during the last years thanks to better detectors, shorter scan time and iterative image reconstruction). Dual-energy CT improves capabilities of this modality for soft-tissue contrast and makes the analysis of MDE feasible. The rare case of a combination of apical HCM and rare congenital coronary anomaly depicts high diagnostic value of modern imaging modalities (first of all, CT and MRI) in difficult diagnostic situations.
- Villa AD, Sammut E, Nair A, Rajani R, Bonamini R, et al. (2016) Coronary artery anomalies overview: The normal and the abnormal. World J Radiol 8: 537-555. [Crossref]
- Angelini P, Velasco JA, Flamm S (2002) Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation 105: 2449-2454. [Crossref]
- Basso C, Maron BJ, Corrado D, Thiene G (2000) Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol 35: 1493-1501. [Crossref]
- Erol C, Seker M (2011) Coronary Artery Anomalies: The Prevalence of Origination, Course, and Termination Anomalies of Coronary Arteries Detected by 64-Detector Computed Tomography Coronary Angiography. J Comput Assist Tomogr 35: 618–624. [Crossref]
- Clemente A, Del Borrello M, Greco P, Mannella P, Di Gregorio F, et al. (2010) Anomalous origin of the coronary arteries in children: diagnostic role of three-dimensional coronary MR angiography. Clin Imaging 34: 337–343. [Crossref]
- Hansen MW, Merchant N (2007) MRI of Hypertrophic Cardiomyopathy: Differential Diagnosis, Risk Stratification, and Posttreatment MRI Appearances. AJR Am J Roentgenol 189: 1344-1352. [Crossref]
- Noureldin RA, Liu S, Nacif MS, Judge DP, Halushka MK, et al. (2012) The diagnosis of hypertrophic cardiomyopathy by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14: 17. [Crossref]
- Ibrahim T, Schwaiger M (2000) Diagnosis of apical hypertrophic cardiomyopathy using magnetic resonance imaging. Heart 83: E1. [Crossref]
- Stainback RF (2012) Apical hypertrophic cardiomyopathy. Tex Heart Inst J 39: 747-749. [Crossref]
- Ghersin E, Lessick J, Litmanovich D, Engel A, Reisner S (2006) Comprehensive multidetector CT assessment of apical hypertrophic cardiomyopathy. Br J Radiol 79: e200-204. [Crossref]
- Eriksson MJ, Sonnenberg B, Woo A, Rakowski P, Parker TG, et al. (2002) Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol 39: 638-645. [Crossref]







