Many scientists have been looking for faster, more accurate and confirmatory biomarkers of acute myocardial infarction (AMI). The aim has been to investigate an assay for the determination of perilipin-2 in human serum, and to check its clinical relevance as a marker of acute myocardial infarction (AMI First type). We have analytically and clinically examined a sandwich ELISA for perilipin-2 measurement. The Perilipin-2 serum level did not differentiate between men and women, but individuals with AMI showed to have higher concentrations of this marker. Perilipin-2 correlated with the serum TNF-a, ultra-sensitive (us) CRP, cystatinC and negatively with Apolipoprotein A1, HDL cholesterol and adiponectin. Using ROC analysis, it was found that the diagnostic efficiency of perilipin-2 was (excellent) and significantly higher than the others. We conclude that perilipin-2 is a very promising biomarker for the diagnosis of acute coronary syndrome
perilipin-2, acute coronary syndrome, ELISA, AMI First type
Cardiac biomarkers are usually used for the diagnosis of myocardial infarction (usually troponin cardiac T or I (cTn.). A large proportion of patients diagnosed with unstable angina were found to have elevated levels of cTn, and those patients were found to have a worse prognosis compared to those who did not have elevated levels of cTn [3-5].
Despite the fact that the determination of the cTn is deemed an accurate and correct indicator, it does not always mean an increase in the diagnosis of AMI. Therefore, many research groups are looking for faster, more accurate and confirmatory biomarkers of AMI or coronary artery disease [1].
Optimum indicator of myocardial ischemia without necrosis, yet while not described there’s only scanty information about the ultrasensitive determination of cTn (T or I), Albumin cobalt binding (ACB), Heart – Fatty acid bounding protein (H-FABP), Glycogen phosphorylase I (GPBB–I), etc.; In addition, it is not recommended to measure myoglobin for its low diagnostic efficiency. Recommended diagnostic schemes are usually based on serial determination of troponin. They have a broad diagnostic window, but the time to onset may not be optimal for the detection of small lesions and their etiology. However, recent information described AMI first type and second type reasons [5].
One of the potential biomarkers could be perilipin-2. Perilipin-2 (Plin2), also known as adipose differentiation-related protein (ADRP), or adipophilin, is a member of the PAT family involved in lipid droplet (LD) formation in the liver and peripheral tissues. Although Plin2 was originally identified as a highly expressed gene in adipocytes, its physiological role in mature adipocytes is largely unknown. A recent report describes that Plin2 mRNA levels increased during adipocyte differentiation whereas the protein levels did not.
Recent papers also suggest that fat might accumulate around the heart in epicardial adipose tissue or inside the heart as lipid droplets (LDs). This process is called myocardial steatosis [1]. It’s known from some studies that immunochemistry for perilipin (Plin) 1 and 2 was used to characterize LDs and their localization in adipocytes or myocardial cells, respectively.
Myocardial steatosis is greater in CAD than non-CAD subjects, that dependes on both the metabolically active adipocytes interspersed among cardiomyocytes and the higher fat deposition inside cardiomyocytes. Serum adiponectin and waist circumference are independent predictors of myocardial steatosis [1].
This means that PLin1 and PLin2 are significantly higher in subjects with coronary artery disease than in non-CAD subjects, as did apoptosis. PLin was positively associated with circulating leptin, high-sensitivity C-reactive protein, and apoptosis, and negatively with adiponectin. PLin2 was positively associated with the body mass index, waist circumference, and leptin and negatively with adiponectin [1].
Also, epatocellular steatosis is the most frequent liver disease in the western world and may develop further to steatohepatitis, liver cirrhosis and hepatocellular carcinoma. It was previously shown that lipid droplet (LD)-associated proteins of the perilipin family are expressed differently in hepatocyte steatosis and that perilipin is expressed de novo. LD-maturation in hepatocytes in vivo and in vitro involves expression of perilipin. Therefore, perilipin might be used for the differential diagnosis of chronic vs. acute steatosis [2].
Other results suggest that Plin2 is degraded in the cytosol in its N-terminal amino acid sequence-dependent manner and instead becomes stable when localized on LDs. The scientists´ findings highlight the relationship between protein stability and an unnoticed function of Plin2 during lipolysis in adipocytes [7].
This year, an interesting paper was presented about the effects of perilipin 2 in the area of atheroprotection [10].
To validate the reliability of the assay, we tested the precision and the accuracy. To analyze the spiking recovery, human serum samples from three subjects with baseline perilipin-2 levels of 17.1, 20.2 and 13.0 Ng/l were spiked with increasing amounts of recombinant protein (+12.5, +25 and +50 Ng/l) and assayed. The mean recovery was 99.0 %. Moreover, we tested human serum samples from another three subjects with baseline perilpin-2 levels of 33.5, 69.4 and 62.4 Ng/l for dilution linearity. The mean recovery was 103 %.
The limit of detection of the assay was 0.2 Ng/l and the limit of quantification (analytical limit of detection multiplied by a sample dilution) was 1.4 Ng/l; the intra-assay coefficient of variation (CV) was less than 5 % and the inter-assay coefficient of variation (CV) were always less than 23 %.
Study subjects
The Ethics Commission of the Hospital Šternberk, Czech Republic, approved the study. A total of 29 individuals with suspicion for acute coronary syndrome after elimination type 2 of acute myocadial infarction were examined. The criteria of type 1 and 2 of AMI were taken from the paper of Collinson (2015) [5].
Sampling and data measurement
Anthropometric (height, weight, BMI, clinical (systolic and diastolic pressures)) and laboratory fasting biomarkers were performed. Blood samples were drawn under aseptic precautions from vena cubiti after a several-minute rest in the half-sitting position. Serum samples were separated in a cooled centrifuge at 3000 g for 20 minutes and immediately analyzed for total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, high sensitivity CRP, cystatin C, adiponectin, (all Biovendor, Advia 1650, Teritown, Siemens), TNF-a (Immulite 2000, Siemens). The perilipin-2 serum level was determined by the assay presented above and an adiponectin serum level was determined by a commercially available ELISA kit (Biovendor, DX2, Dynatech) in serum samples stored at -80° C.
We also performed a methacetin breath test for the diagnosis of the level of liver damage.
Statistical analysis
The data obtained was processed by means of the software Medcalc (Medcalc, Mariakerke, Belgium). The value p<0.05 was considered as statistically significant. Because of anomalous data distribution in evaluated parameters, Spearman’s correlation coefficients were used to establish the association between perilipin–2 levels and the other parameters. The comparisons of perilipin-2 serum values between subjects with and without AMI were performed by either the Student’s t test or Mann-Whitney test according to the data distribution. All the data was presented as medians and/or means ± standard deviation.
The study analyzed 29 probands, of which 15 were defined as AMI first type, while 14 probands showed no signs of AMI (both types). We did not observe any significant difference in serum perilipin-2 between the tested men and women (54 years, p=0.6). In all probands, us-CRP and cystatin C (Biovendor), cTnI and TNF-alpha (LEIA Siemens), ApoAI, HDLcholesterol and adiponectin (Biovendor) were measured up to 3 hours after admission or earlier before chestpain.
Individuals with AMI had higher concentrations of perilipin-2 (P<0.01) us-CRP (P<0.01), TNF-a (P=0.02), cystatin C (P< 0.01 ) (Table 1).
Table 1. Values of measured parameters according to diagnosis 1 type of AMI
|
AMI
|
|
|
|
Non AMI
|
|
|
|
|
|
Biomarker
|
X
|
Median
|
SD
|
Xxxxx
|
X
|
Median
|
SD
|
Differences
|
|
Perilipin-2
|
22.1
|
23
|
7
|
|
13.2
|
14.7
|
6
|
P<0.01
|
|
TNF-alpha
|
16.7
|
14.0
|
6.0
|
|
27.8
|
25.8
|
10
|
P=0.02
|
|
Cystatin C
|
6.7
|
5
|
1.2
|
|
3.8
|
3.9
|
1.0
|
P=0.04
|
|
myoglobin
|
54
|
43
|
12.5
|
|
39
|
22
|
18
|
P=0.02
|
|
hsCRP
|
29.4
|
20,1
|
13
|
|
16.7
|
12.5
|
6
|
P<0.01
|
|
ApoA-1
|
0.9
|
0.8
|
0.3
|
|
1,4
|
1,5
|
0.3
|
P=0.02
|
|
HDL
|
0,6
|
0,7
|
0,4
|
|
1,2
|
1,2
|
0.3
|
P=0.3
|
|
Adiponetin
|
5
|
7
|
5
|
|
15.6
|
13
|
3.6
|
P=0.1
|
|
Omentin-1
|
4.4
|
5.7
|
6
|
|
12.5
|
16
|
4.2
|
P=0.1
|
Serum perilipin-2 correlated with cystatin C (r=0.56), TNF-a (r=0.32) us-CRP (r=0.41), and negatively correlated with HDL cholesterol (r=-0.52), adiponectin (r=-0.59), and omentin-1 (r=-0.6). No significant correlation was found between serum periliprin-2 and BMI, blood pressure, triglycerides and total cholesterol (data not shown).
We have also confirmed a link between hepatic steatosis (breath test with methacetin) and values of perilipin-2 (data not shown).
In 15 patients with AMI we have not confirmed signs of pulmonary embolia, arrythmia, anemia, global hypoxia, myocardial damage other etiology than acute myocardial ischemia (they have AMI first type).
Using ROC analysis, it was found that the diagnostic efficiency of perilipin-2 was excellent, and significantly higher than the efficiency for myoglobin or us-CRP or other parameters (AUC 1, sensitivity 100%, specificity of 100%; the cut-off=15 ng/l, AUC of myoglobin was 0.78 and us-CRP 0.64 (Figure 1).
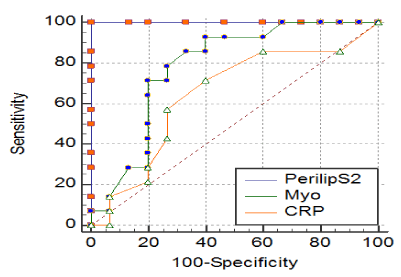
Figure 1. AUC for perilipin-2 was 1 (red line). Sensitivity was 100%, specificity was 100%, cit-off value was 15 ng/l of perilipin-2 in serum. AUC for myoglobin was 0.78 (green line), AUC for hsCRP was 0.64 (orange line).
Abbreviations: perilipS2 – perilipin – 2, myo – myoglobin, CRP –us CRP
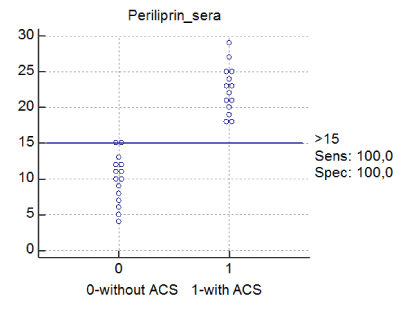
Figure 2. Dot graph for periliprin-2 and mentioned diagnosis Abbreviations: periliprin= perilipin -2. 0- without AMI (First or second type), 1-with type 1 of AMI. Periliprin-2 is described ng/l.
The definition of type 2 of AMI is unsatisfactory, as it is not really defined by what it is but rather what it is not. Nevertheless, the clinical problem remains: to distinguish acute MI (associated with plaque rupture or erosion, traditional or type 1 MI)—for which there is evidence-based treatment—from acute secondary ischaemic cardiac injury, including type 2 MI, where such treatments are not evidence based and may even be harmful [3].
The role of troponin measurement is in the differential diagnosis of suspected non-ST-segment elevation MI (NSTEMI). Patients presenting with ST-segment elevation MI require an immediate intervention based on current guidelines and thus should not wait for troponin results.
The first stage is to assess the probability that the patient has underlying acute coronary artery disease (CAD) from their clinical history and that the symptoms are caused by cardiac ischemia. It is important that the results of the laboratory tests taken as a whole are consistent with the clinical features and the ECG. If the troponin is not elevated, then a repeat of the test is required before acute myocardial injury can be excluded with certainty. On the other hand, a single elevated troponin is not, on its own, diagnostic of AMI. However, an elevated troponin along with other appropriate clinical and laboratory evidence raises the probability that the diagnosis is NSTEMI. The higher the troponin value, the greater the probability that the final diagnosis will be AMI. It must be stressed that the data must be consistent. An elevated troponin plus a normal ECG or nonspecific changes should immediately raise suspicion of an alternate diagnosis. Similarly, there should be no other reasons of causes of minor troponin elevation, such as renal failure or significant age. Other potential acute causes of troponin elevation associated with CAD, such as tachycardia or other conditions causing increased oxygen demand or reduced oxygen supply, indicate a type 2 MI. However, even if the clinical probability of underlying CAD is high, a final diagnosis of MI requires demonstration of a changing troponin value. Contemporary sensitive assays are able to detect a significant troponin change on retesting if the initial value is elevated with a repeat test performed 2-3 hours later and with high sensitivity assays within 1 hour.
2021 Copyright OAT. All rights reserv
Repeat testing allows the immediate distinction between an acute myocardial injury and underlying chronic myocardial damage causing troponin elevation. This resolves the dilemma of the patient with renal disease (or old age). An elevated troponin in the first sample is to be expected in the patient with renal failure. A changing troponin indicates acute injury and may be due to an acute MI. In contrast, an elevated troponin that does not change significantly is due to chronic myocardial injury and may require further investigation but possibly not as an in-patient and not necessarily by a cardiologist.
The most important factor is to be aware that other clinical conditions can cause a troponin elevation. Troponin elevation is specific for myocardial injury, but not every troponin elevation is an MI. The presence of other clinical conditions such as pneumonia or pulmonary embolus should shift the clinical focus to an appreciation that the troponin elevation is an additional prognostic rather than diagnostic finding. If there is diagnostic uncertainty, then cardiac imaging, either invasive or noninvasive, as well as other types of cross sectional imaging is necessary to provide additional information.
Therefore, the key features to diagnose a type 2 MI (more properly secondary ischaemic cardiac injury), can be summarised as follows:
- An elevated but changing troponin value
- Clinical features inconsistent with type 1 acute MI
- Clinical conditions known to increase the oxygen demand or decrease the oxygen supply lik tachycardia
- Potentially confounding clinical conditions or comorbidities that are potentially associated or known to be associated with myocardial injury
- Absence of symptoms and/or signs indicating other non-ischemic causes of troponin elevations like myocarditis.
Treatment of type 2 MI is to treat the underlying condition and hence remove the cardiac insult. To adequately assess the prognosis and determine the appropriate further treatment in patients with type 2 MI, information about whether the patient has (or is likely to have) significant underlying CAD is essential. In addition, it is important to remember that elevated troponin in the patient with non-acute MI is not redundant information but also indicates an adverse prognosis [3-5]. This knowledge is a reason to search for new, faster and more accurate biomarkers of the presence of myocardial infarction [5].
The cytoplasmic lipid droplet (LD) protein perilipin-2 is expressed in multiple nonadipose tissues, where it is thought to play a role in regulating their lipid storage properties. Recent papers demonstrated that the absence of perilipin-2 prevents high-fat diet-induced obesity in male and female mice. This response is associated with increased formation of subcutaneous beige adipocyte cells with uncoupling protein 1 expression, and amelioration of inflammatory foci formation in white adipose tissue and steatosis in the liver. Experiments demonstrate that perilipin-2 loss results in reduced energy intake and increased physical activity in response to high fat diet feeding. The authors` study provides the first evidence that perilipin-2 contributes to high fatty diet-induced obesity by modulating food intake, and that its absence prevents obesity-associated adipose tissue inflammatory foci and liver steatosis [6].
Cytosolic lipid droplets (LDs) are present in most cell types, and consist of a core comprising neutral lipids, mainly triglycerides and sterol esters, surrounded by a monolayer of phospholipids. LDs are heterogeneous in their structure, chemical composition, and tissue distribution. Several proteins, including perilipins and other structural proteins, lipogenic enzymes, lipases and membrane-trafficking proteins, coat LDs. Evidence suggests that LD proteins are involved in the pathophysiology of fatty liver diseases characterized by excessive lipid accumulation in hepatocytes. Three typical pathogenesis of chronic liver conditions with LD pathophysiology are: hepatitis C virus infection, non-alcoholic fatty liver disease (NAFLD), and alcoholic liver disease [7,8].
Recently, it was described that the potential role of perilipin-2 in a therapy of NAFLD [9]. In other studies, authors confirmed that Plin2 liver-specific ablation alleviates diet-induced hepatic steatosis and inflammation. Plin2 is a target for NAFLD therapy [11].
In another study, which aim was to evaluate the effect of the Ser251Pro mutation of PLIN2 gene in a cohort with a higher predisposition to obesity-associated metabolic alterations, scientists observed a strong significant association between the PLIN2 Pro251 mutation and lower insulin secretion associated with an increased insulin-sensitivity. This mutation is negative risk factor for metabolic syndrome and type 2 diabetes mellitus [12].
We know that promoting reverse cholesterol transport from the arteries can ameliorate atherosclerosis development. The process involves cholesterol efflux from foam cells to extracellular acceptors such as apolipoprotein A-I and HDL cholesterol that mediates transport to the liver. As previously described, Perilipin-2 is a lipid droplet (LD)-associated protein that, in macrophages, facilitates cholesterol storage and prevents efflux. A recently published article hypothesized that atheroprotection would be enhanced by concurrently targeting PLin2 to increase the efflux capacity of foam cells and increasing plasma apoA-I and HDL cholesterol. This paper demonstrates a mutually beneficial relationship between PLin2 deficiency and elevated apoA-I/HDL cholesterol in preventing atherosclerosis development. Scientists hypothesizing that targeting foam cell components to mobilize cholesterol may be a promising strategy to enhance the atheroprotection of plasma cholesterol acceptors [10].
The mentioned results could be with our findings (we are aware that our study was in the pilot mode) support hypothesis about the potential use of perilipin-2 in early and exact diagnostics of acute myocardial infarction and its prognosis. This is indicated as a new approach for the diagnosis in the mentioned disease.
In conclusion, it seems that the determination of perilipin-2 could be an entirely new metabolic marker for the presence of First type of AMI with unexpectedly and extraordinarily high diagnostic efficiency.
As a major limitation of the study can be considered a small number of measured probands. But it was a pilot study in which we would like to continue and at the same time inform professionals about our interesting results and the new conception of entry of this diagnosis.
- Mazzali G, Fantin F, Zoico E, Sepe A, Bambace C, et al. (2015) Heart Fat Infiltration In Subjects With and Without Coronary Artery Disease. J Clin Endocrinol Metab 100: 3364-3371. [Crossref]
- Pawella LM, Hashani M, Eiteneuer E, Renner M, Bartenschlager R2, et al. (2014) Perilipin discerns chronic from acute hepatocellular steatosis. J Hepatol 60: 633-642. [Crossref]
- Collinson P, Lindahl B2 (2015) Type 2 myocardial infarction: the chimaera of cardiology? Heart 101: 1697-1703. [Crossref]
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, et al. (2012) Third universal definition of myocardial infarction. Eur Heart J 33: 2551-2567. [Crossref]
- Collinson PO, Garrison L, Christenson RH (2015) Cardiac biomarkers - A short biography. Clin Biochem 48: 197-200. [Crossref]
- McManaman JL, Bales ES, Orlicky DJ, Jackman M, MacLean PS, et al. (2013) Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res 54: 1346-1359. [Crossref]
- Takahashi Y, Shinoda A, Kamada H2,3, Shimizu M, et al. (2016) Perilipin2 plays a positive role in adipocytes during lipolysis by escaping proteasomal degradation. Sci Rep 6: 20975. [Crossref]
- Carr RM, Ahima RS (2015) Pathophysiology of lipid droplet proteins in liver diseases. Exp Cell Res 340: 187-192. [Crossref]
- Goh VJ, Silver DL (2013) The lipid droplet as a potential therapeutic target in NAFLD. Semin Liver Dis 33: 312-320. [Crossref]
- Son SH, Goo YH, Choi M, Saha PK, Oka K, et al. (2016) Enhanced atheroprotection and lesion remodelling by targeting the foam cell and increasing plasma cholesterol acceptors. Cardiovasc Res 109: 294-304. [Crossref]
- Najt CP, Senthivinayagam S, Aljazi MB, Fader KA, Olenic SD1\, et al. (2016) Liver-specific loss of Perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. Am J Physiol Gastrointest Liver Physiol 310: G726-738. [Crossref]
- Sentinelli F, Capoccia D, Incani M, Bertoccini L, Severino A, et al. (2015) The Perilipin 2 (PLIN2) gene Ser251Pro missense mutation is associated with reduced insulin secretion and increased insulin sensitivity in Italian obese subjects. Diabetes Metab Res Rev. [Crossref]


