Of the proposed causes and associations of nonuremic calciphylaxis, IgA is not included. We encountered a 60-year-old Hispanic female with stage III chronic kidney disease secondary to IgA nephropathy, who presented with a painful abdominal eschar. The diagnosis of nonuremic calciphylaxis was made, with IgA nephropathy being the most interesting potential cause. Our case represents the first report of nonuremic calciphylaxis in a patient with IgA nephropathy, prompting us to propose a relationship that is independent of uremia.
calciphylaxis, nonuremic, uremia, IgA nephropathy, nephrotic syndrome, hypercoagulable, chronic kidney disease, end stage renal disease
Calciphylaxis occurs when ischemia and skin necrosis develop secondary to vasculature calcification. Most commonly, calciphylaxis is seen in the setting of End Stage Renal Disease (ESRD) requiring renal replacement therapy; this situation is termed uremic calciphylaxis. This is in contrast to nonuremic calciphylaxis, which represents the vast minority of cases and occurs in individuals not requiring renal replacement therapy. Nonuremic causes of calciphylaxis are rare. Nigwekar et al. performed a systematic review of thirty-six patients with histopathologically-confirmed calciphylaxis in the absence of ESRD, severe Chronic Kidney Disease (CKD) with a serum creatinine >3 mg/dL or creatinine clearance <15 millimeters per minute, acute kidney injury requiring renal replacement therapy, or renal transplantation [1]. Their review revealed that the most common primary causes of nonuremic calciphylaxis were hyperparathyroidism (27.8% of cases), malignancy (22.2%), alcoholic liver disease (16.7%), connective tissue disease (11.1%), diabetes (5.5%), chemotherapy-induced protein C and S deficiency (2.8%), Crohn’s disease (2.8%), osteomalacia treated with nadroparin calcium (2.8%), POEMS syndrome (2.8%), vitamin D deficiency (2.8%), weight loss (2.8%), and CKD (but not ESRD) (2.8%) [1]. Other predisposing factors or associations included corticosteroid use, warfarin use, albumin or blood transfusions, protein C or S deficiency, and precipitating trauma leading to cutaneous lesions [1]. Kalajian et al. analyzed thirteen cases of calciphylaxis in patients with normal renal and parathyroid function and devised a list of potential risk factors for the development of calciphylaxis in nontraditional patients [2]. Their list supports the findings of Nigwekar et al. and also adds hypoalbuminemia (78% of patients), systemic inflammation (29%), liver cirrhosis (21%), obesity (14%), and infection (7%) as potential risk factors [2]. It has become increasingly apparent that a wide variety of conditions may be intimately involved in the development of calciphylaxis.
A 60-year-old Hispanic female presented with a 3-month history of multiple painful dusky indurated plaques and necrotic stellate eschars located on the legs and abdomen. Relevant past medical history included CKD stage III attributed to biopsy-proven IgA nephropathy with no dialysis history, diabetes mellitus type II, and prior uterine cancer treated with chemotherapy and radiation. Laboratories confirmed a stable BUN and creatinine (insignificant change from baseline). Phosphorus, calcium, and albumin were within normal limits. Previous labs revealed an ANA <1:80, ANCA <1:20, normal IgG and IgG subclass levels, and negative cryoglobulins. Physical examination was significant for a twelve-centimeter well-defined eschar located on the anterior abdomen (Figure 1) and multiple discrete, dusky brown to purple, well-defined, discoid, indurated plaques located on the lower extremities. Both the plaques and the eschar were exquisitely tender to palpation. Apunch biopsy showed perivascular calcification in the superficial and deep dermis, intimal fibrosis, and diffuse dystrophic calcification (Figures 2a and 2b). As the patient never required renal replacement therapy, the clinical and histopathological features were supportive of nonuremic calciphylaxis. The patient was placed on antibiotics for concern of secondary infection and was started on Cinacalcet 30 mg daily. A consulting service surgically debrided the abdominal eschar and the patient was discharged with a wound vacuum. To our knowledge, the patient remains alive but has required multiple hospitalizations since her initial presentation.
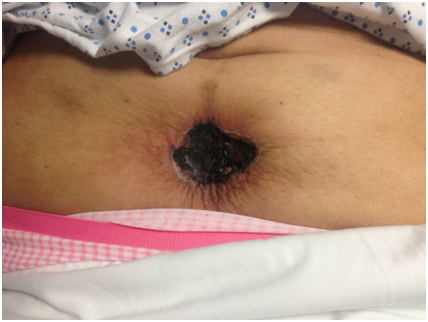
Figure 1. Calciphylaxis of the lower abdomen.
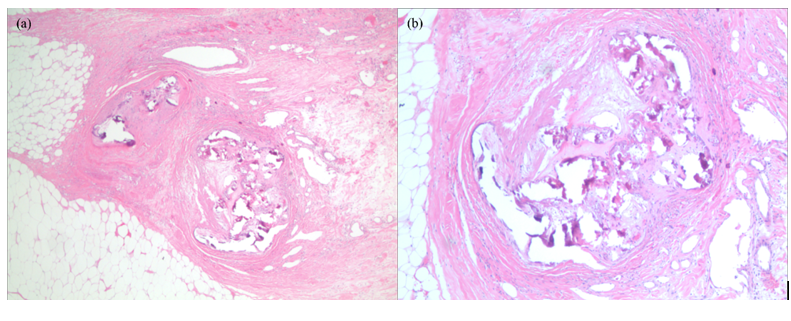
Figure 2. Histological images of the patient’s abdominal eschar. There is calcification of the intima and media of small- to medium-sized vessels. Both images are of the same affected vessels. Hematoxylin and eosin, original magnification (a) 2x; (b) 4x.
Ultimately a diagnosis of nonuremic calciphylaxis was applied to our patient. Though our patient did not have entirely normal renal function, she also failed to meet Nigwekar et al.’s classification for uremic calciphylaxis [1]. Based on her non-traditional presentation of calciphylaxis, we have associated the risk factors described by Kalajian et al. with our patient’s development of nonuremic calciphylaxis. Our patient had multiple diagnoses that are recognized as causative or associated with a diagnosis of nonuremic or non-traditional calciphylaxis. These diagnoses include a history of malignancy (uterine cancer) treated with chemotherapy and radiation, diabetes, CKD (with no history of renal replacement therapy), preceding trauma (radiation leading to a radiation burn to her central abdomen), hypoalbuminemia (her albumin ranged from 2.2 to 3.7 g/dL), and obesity (BMI 48 kg/m2). Based on these factors, along with her histopathologically-supported calciphylaxis in the absence of the aforementioned factors, a diagnosis of nonuremic calciphylaxis is appropriate.
Our patient’s history of IgA nephropathyis of most interest. There have been several reports of calciphylaxis in patients with renal disease secondary to IgA nephropathy (Table 1) [3-6]. However all of these patients required renal replacement therapy, whether in the form of dialysis or kidney transplant. None of these reports makes an association between IgA nephropathy and calciphylaxis, likely because all included cases of uremic calciphylaxis only. Since our patient had IgA nephropathy and nonuremic calciphylaxis, we question whether IgA nephropathy may be in some way related to the development of calciphylaxis.
Table 1. Summary of published reports describing patients with calciphylaxis and IgA nephropathy.
|
Source
|
Age/
Sex
|
Cause
|
Renal Replacement Therapy?
|
Labs
|
Treatment
|
Outcome
|
|
Our patient
|
60/F
|
CKD stage III due to IgA nephropathy
|
No
|
Calcium 9.3 mg/dL, phosphorus 4.6 mg/dL, creatinine 3.2 mg/dL
|
Antibiotics, surgical debridement, and Cinacalcet
|
Improved
|
|
Timmis and Morgan [3]
|
17/M
|
CKD stage V due to IgA nephropathy
|
Yes (dialysis)
|
Calcium 2.56 mmol/L, phosphorus 3.32 mmol/L, PTH 1.95 pmol/L
|
Dialysis, cessation of vitamin D analogues and calcium-based phosphate binders, Cinacalcet
|
Improved
|
|
Sanchez et al. [4]
|
66/F
|
Kidney transplant secondary to mesangial glomerulonephritis caused by IgA deposits
|
Yes (kidney transplant)
|
Calcium 8.6 mg/dl, phosphorous 6.6 mg/dl, PTH 653 pg/mL, creatinine 2.9 mg/dL
|
Cinacalcet 30 mg/day and aluminum hydroxide as a phosphorus binder
|
Death secondary to cardiac arrest
|
|
Hanvesakul et al. [5]
|
70/M
|
Kidney transplant secondary to end stage renal failure from IgA nephropathy
|
Yes (dialysis and kidney transplant)
|
Normal calcium, phosphorus, and PTH
|
IV antibiotic for superinfection; discontinuation of calcium supplements
|
Full resolution of lesions
|
|
Mathur et al. [6]
|
58/M
|
Renal failure due to mesangioproliferative glomerulonephritis secondary to IgA nephropathy
|
Yes (dialysis)
|
Calcium 2.02 mmol/L, phosphorus 2.59 mmol/L, PTH 248 pg/mL
|
Local wound care with debridement and grafting
|
Death secondary to septicemia and spontaneous gastrointestinal hemorrhage
|
Thrombosis can occur in patients with nephrotic syndrome as a result of a lack of balance between factors involved in anticoagulation (such as antithrombin III, protein C, and protein S) and those involved in procoagulation (such as fibrinogen, factor V, factor VIII, von Willebrand factor, and fibrinolytic system and plasminogen activator inhibitor), as well as due to abnormalities in platelet activation [7]. Breakdown of the glomerular capillary wall selectivity barrier leads to a leakage of antithrombotic factors in the urine, which is counterbalanced by an increase in plasma procoagulant factors [8]. Thrombosis may occur from preferential loss of the proteins that are involved in inhibiting the clotting cascade, from increased synthesis of pro-thrombotic factors, or from locally activating the glomerular hemostasis system [9]. Furthermore, risk factors for a hypercoagulable state in cases of nephrotic syndrome include several of the same risk factors for calciphylaxis, such as obesity, hypoalbuminemia, and the use of steroids [7].
A review of the primary literature of patients with calciphylaxis revealed that 38% had decreased protein C levels, and 43% had decreased protein S levels [10]. These researchers concluded that there was sufficient evidence to suggest that a hypercoaguable state does play some role in the pathogenesis of calciphylaxis, further supported by the findings of healed skin lesions after treatment with low molecular weight heparin or tissue plasminogen activator. We suggest the idea of an association between calciphylaxis and IgA nephropathy secondary to a common pathophysiology. Both conditionsare vascular occlusive diseases that can result in the formation of ahypercoagulable state. This implication would link IgA nephropathy and other nephrotic syndromes as the cause of ESRD associated with the development of calciphylaxis.
As our patient possessed multiple risk factors for the development of nonuremic calciphylaxis, we agree that it is impossible to definitively attribute IgA nephropathy as the underlying cause. However her known history of IgA nephropathy prompted us to question if this condition could be generally related to nonuremic calciphylaxis. Our proposed pathophysiologic explanation for this association supports the recent attention devoted to hypercoagulability’s role in the development of calciphylaxis. We anxiously await any future publications describing patients of IgA nephropathy and nonuremic calciphylaxis who lack additional risk factors for this condition, as this would support our theory. Whether or not our patient represents the first case of such a situation, our proposed association and conclusions may ultimately lead to a better understanding of calciphylaxis.
The authors would like to thank Dr. Sean Smith for his support in the production of this manuscript.
2021 Copyright OAT. All rights reserv
- Nigwekar SU, Wolf M, Sterns RH, Hix JK (2008) Calciphylaxis from nonuremic causes: A systematic review. Clin J Am Soc Nephrol 3: 1139-1143. [Crossref]
- Kalajian AH, Malhotra PS, Callen JP, Parker LP (2009) Calciphylaxis with normal renal and parathyroid function: not as rare as previously believed. Arch Dermatol 145: 451-458. [Crossref]
- Timmis A, Morgan H (2010) Calciphylaxis in a paediatric patient. BMJ Case Reports. [Crossref]
- Hanvesakul R, Silva MA, Hejmadi R, Mellor S, Ready AR, et al. (2009) Calciphylaxis following kidney transplantation: a case report. J Med Case Rep 3: 9297. [Crossref]
- Sánchez MP, Pérez MC, Lorman RS, Borroy CS (2009) Proximal calciphylaxis in a liver and kidney transplant patient. Nefrología 29: 489-490. [Crossref]
- Mathur RV, Shortland JR, El Nahas AM (2001) Calciphylaxis with facial involvement. Nephrol Dial Transplant 16: 2256-2257. [Crossref]
- Barbano B, Gigante A, Amoroso A, Cianci R (2013) Thrombosis in nephrotic syndrome. Semin Thromb Hemost 39: 469-476.
- Charlesworth JA, Gracey DM, Pussell BA (2008) Adult nephrotic syndrome: non-specific strategies for treatment. Nephrology (Carlton) 13: 45–50. [Crossref]
- Llach F (1985) Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney Int 28: 429-439. [Crossref]
- Harris RJ, Cropley TG (2011) Possible role of hypercoagulability in calciphylaxis: review of the literature. J Am Acad Dermatol 64: 405-412. [Crossref]


