Abstract
Vulvar and vaginal melanomas are infrequent malignancies characterized by an aggressive behaviour and a poor prognosis. Surgery is the treatment of choice for primary tumors and loco-regional metastases. As target therapies have emerged as effective treatments both in the advanced and adjuvant settings, molecular profiling of primary tumors and/or metastases is mandatory in order to choose the proper treatment in melanoma patients harbouring specific mutations. Recently, some studies have analyzed the molecular profile of urogenital melanomas, but limited to few specimens and ending with a lack of general agreement. We evaluated a cohort of vulvar and vaginal melanomas for clinical-pathological parameters and molecular profile of the most frequently mutated genes in melanomas, KIT, BRAF and NRAS. Our study population was composed of 23 cases, including 14 vulvar, 7 vaginal and 2 cases of concomitant vulvar and vaginal melanomas. We analyzed clinical-pathological parameters such as histotype, number of mitoses, presence of ulceration, regression, vascular invasion and metastasis, in addition to recurrence and overall survival. Molecular analyses were performed by bi-directional Sanger sequencing for KIT (exons 9, 11, 13 and 17), BRAF (exons 11 and 15) and NRAS (exons 2 and 3). We applied multivariate models to assess the associations of KIT, BRAF and NRAS status with prognostic factors, adjusting for age. KIT mutations were found in 4 samples (17%), 3 vulvar and 1 vaginal melanoma. NRAS alterations were observed in 2 of 15 available samples (13%) equally distributed between the two categories; BRAF mutations were detected in 1 vulvar melanoma of 19 available samples (5%). KIT mutation was associated with significantly lower number of mitosis and lower Breslow thickness (P=0.01 and P=0.05, respectively, adjusting for age and site). Thicker melanomas were found to be associated with a worse progression free and overall survival (P=0.01 and P=0.03, respectively, adjusting for age). In conclusion in our cohort, melanomas of female genital tract harboured mutations in all the three genes tested, with KIT and NRAS most commonly mutated. KIT status was found to be significantly associated with prognostic factors. Since melanoma patients carrying mutations of KIT, NRAS or BRAF might benefit from target therapy such as imatinib, MEK inhibitor or anti-BRAF drugs, we suggest that even in these rare melanomas molecular analysis should be performed in order to drive proper therapeutic treatment.
Key words
Vulvo-vaginal melanomas, BRAF, KIT, NRAS
Introduction
Vaginal and vulvar melanomas are rare tumors, representing 1-3% of melanomas arise in women [1,2] and less than 1% of all female genital tract malignancies [3,4]. Among primary melanomas of the genital tract, vulvar melanomas exhibit the highest frequency, followed by vaginal and cervical. The estimated incidence is 0.1-0.2/100.000 and 0.26-0.46/1.000.000 new diagnosed cases per year of vulvar and vaginal melanomas, respectively [1,2,5,6]. Primary vulvar and vaginal melanomas more frequently occur in Caucasian women in their fifth to seventh decade of life [7-10].
The overall prognosis is poor, with a 5-year survival rate of approximately 47% for primary vulvar melanoma [1] and 18% for primary vaginal melanoma [1,7]. Loco-regional control and survival rates are reported to be related to tumor thickness, ulceration, lymph node spread and age [8,11]. The treatment of choice is surgery [12], but there is lack of consensus about the extent of the surgical approach (i.e., conservative wide local excision versus radical surgery) and the role of the sentinel node biopsy for microscopic staging. The benefıt of adjuvant therapies for higher-risk (i.e., American Joint Committee on Cancer (AJCC) stage II or III) melanomas after surgical excision needs to be further investigated [13,14]. Due to the rarity of these tumors and the lack of evidence-based guidelines for the management of vulvar and vaginal melanoma, these patients are usually treated in accordance to principles of management of cutaneous melanoma [14-16].
Cutaneous melanoma molecular characterization is mainly focused on the MAPK/ERK pathway, with BRAF mutations found in up to 55%-60% of primary tumors arising on intermittently sun-exposed skin, without sun-induced damage [17,18]. However, vulvar and vaginal primary melanomas show a lower rate of BRAF mutations (less than 15%) [17,18] but harbor KIT mutations in up to 40% [19] and NRAS mutations in up to 14% of cases [20]. Recently, some studies on vulvo-vaginal melanomas have shown that KIT aberrations are the most common alterations in vulvar melanomas, while NRAS mutations are more frequently observed in vaginal melanomas [21-25]. Due to the limited number of samples tested in each study and the difficult labelling among vulvar or vaginal category, these findings need to be further investigated.
Nowadays there are active therapies targeting altered genes such as BRAF, KIT or NRAS, with promising results from clinical trials enrolling a subset of patients harboring specific alteration [26-36]. Therefore, extended studies should be performed recruiting vulvo-vaginal melanoma patients, to deeply understand the molecular profile of these lesions, in order to identify patients who may benefits from targeted therapies.
The aim of our study was to evaluate the pathological and molecular features of malignant melanomas of the female genital tract, carefully subdivided into vulvar or vaginal site of onset, in association with prognostic factors and overall survival, in our single-center cohort. We focused on KIT, BRAF and NRAS which are the current targets of molecular therapies in melanomas.
Materials and methods
Patient’s selections
In this study, we evaluated 23 patients with primary vulvar or vaginal melanoma who were diagnosed and treated between 1999 and 2012 at the Istituto Europeo di Oncologia, Milan, Italy. The cohort of patients included 14 vulvar, 7 vaginal and 2 patients with a concomitant vulvar and vaginal primary melanomas. The histopathological classification was performed by two experienced dermatopathologists according to WHO guidelines, evaluating Breslow’s tumor thickness, number of mitoses, presence of ulceration, regression, vascular invasion and metastasis (Figure 1 and Table 1). Consensus was achieved in case of discordance. Complete follow-up was available for 22 patients. All the patients underwent surgical resection with intention to treat; none received target therapies which were not available at the time of diagnosis.
Table 1. Patients and tumor characteristics
| |
|
Frequency
|
%
|
|
Age (Median, q1-q3*)
|
71 (57 - 76)
|
|
|
|
Mitosis (Median, q1-q3*)
|
12 (6 - 15)
|
|
|
|
Breslow (Median, q1-q3*)
|
5 (3 - 9)
|
|
|
|
Site
|
Vagina
|
7
|
30
|
|
Vulva
|
14
|
61
|
|
Vulva and vagina
|
2
|
9
|
|
Histological type
|
Mucous lentiginous melanoma
|
2
|
9
|
|
Nodular melanoma
|
3
|
13
|
|
Superficial spreading melanoma
|
12
|
52
|
|
Polipoid melanoma
|
1
|
4
|
|
Missing
|
5
|
22
|
|
BRAF
|
WT†
|
18
|
78
|
|
pV600
|
1
|
4
|
|
Missing
|
4
|
18
|
|
KIT
|
WT†
|
19
|
83
|
|
p.V560D
|
2
|
9
|
|
p.Y578H
|
1
|
4
|
|
p.N655K
|
1
|
4
|
|
NRAS
|
WT†
|
13
|
56
|
|
p.Q61R
|
2
|
9
|
|
Missing
|
8
|
35
|
|
Ulceration
|
No
|
6
|
26
|
|
Yes
|
16
|
70
|
|
Missing
|
1
|
4
|
*q1-q3: range interquartile; †WT: wild-type
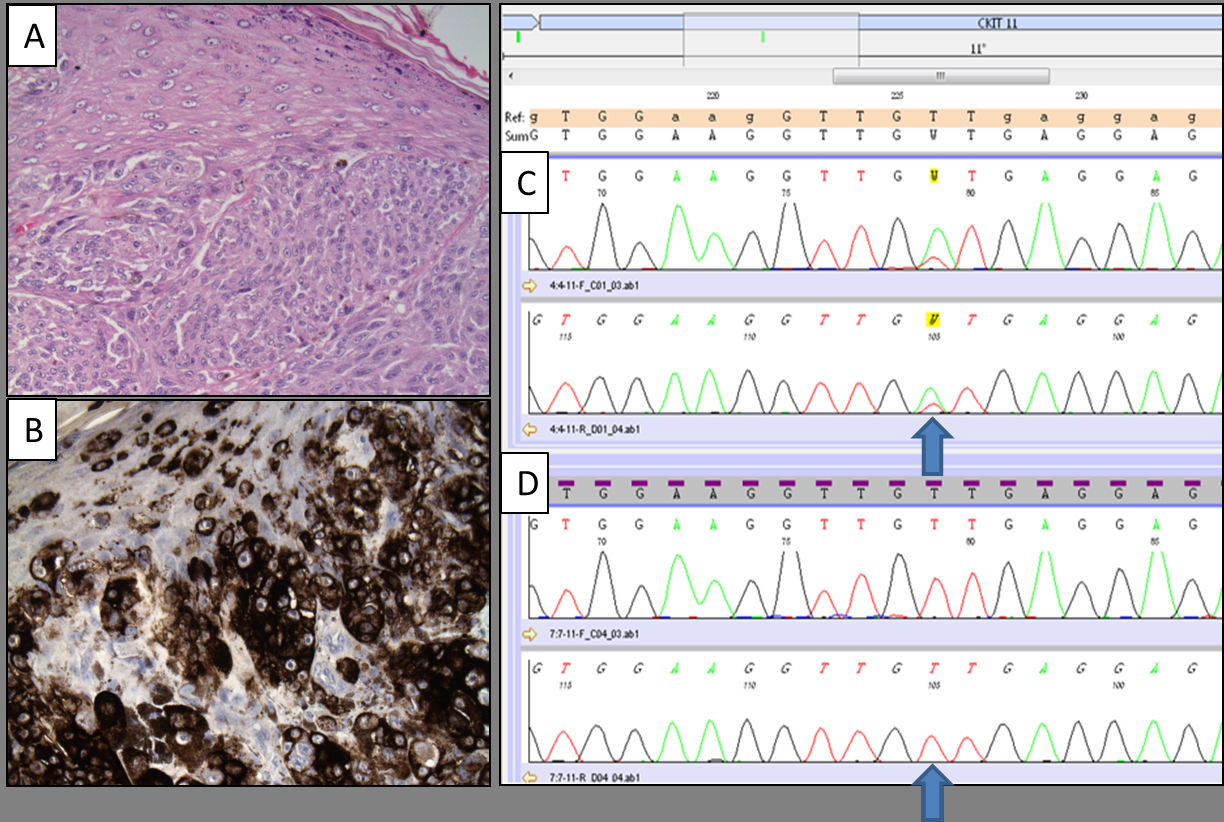
Figure 1. Micrographs showing a detail of vulvar melanoma (A; hematoxylin and eosin stain, original magnification: 200x) displaying strong HMB-45 expression (B; immunohistochemical stain, original magnification: 200x). The mutational analysis of the same cases revealed the presence of KIT exon 11 p.V560D mutation (C; electropherogram image obtained by Bidirectional Sanger Sequencing). Electropherogram image of wild-type sequence of the same gene is reported for comparison (D).
Mutational analyses
KIT (exons 9,11,13 and 17), BRAF (exons 11 and 15) and NRAS (exons 2 and 3) mutational analysis was performed by bi-directional Sanger sequencing. Briefly, the pathologist identified tumor areas with a tumor cellular content of at least 60%; sections of 5-mm thickness were cut from paraffin blocks and the previously identified areas were macrodissected by a sterile scalpel. DNA was extracted using a commercial available kit (QIAamp DNA FFPE Tissue kit, Qiagen, Hiden, Germany), amplified and then purified by enzyme treatment with Exonuclease I and Shrimp Alkaline Phosphatase (GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK). The cycle sequencing reactions were carried out using BigDye Terminator chemistry, followed by removing of unincorporated reagents with BigDye XTerminator kit (Applied Biosystems, Foster City, CA). Sequencing reaction products underwent capillary electrophoresis using 3500Dx Genetic Analyzer (Applied Biosystems, Foster City, CA) and was sequenced on both forward and reverse directions. Mutations were confirmed in two independent amplification runs.
According to the division policy and in order to save tissue, a diagnostic algorithm was applied considering the frequency of the mutations. KIT was the first gene studied and the wild-type cases were subsequently investigated for BRAF and NRAS gene status. In mutated cases, no further analysis was performed due to the mutual exclusivity of these mutations.
Statistical analysis
Descriptive statistics were used to analyze tumor and patients characteristics. Data were reported as relative frequencies (percentage) for categorical variables and median values and interquartile ranges for continuous variables.
We investigated the association of KIT, BRAF and NRAS gene status with melanoma prognostic factors through univariate analyses with Chi-square tests and Wilcoxon rank tests and through multivariate linear models (analysis of covariance, ANCOVA) and linear logistic models, to verify the associations adjusting for confounders.
Overall survival (OS) was calculated from time of diagnosis to death or last follow-up (censored) and Progression Free Survival (PFS) from time of diagnosis to first event (progression or death) or last follow-up (censored). OS curves were estimated with the Kaplan-Meier method. Regression Cox models were used to assess the association of KIT, BRAF and NRAS gene status and prognostic factors with OS or PFS adjusting for confounders.
Since the relative small size of the sample we decided to present P-values from simple multivariate models adjusted for age only. Including more confounders would make models unstable.
For all analyses, two-tailed P<0.05 was considered statistically significant. The statistical analyses were performed with the Statistical Analysis System Version 9.2 (SAS Institute, Cary, NC).
Results
Clinical and pathological features of the overall study cohort (n=23) are summarized in Table 1. The cohort included 14 patients with primary vulvar melanoma, 7 patients with primary vaginal melanoma and 2 patients with a synchronous vulvar and vaginal melanoma.
Pathological features
Data on melanoma histotype were available for 18 patients (Table 1). Superficial spreading melanoma was the most common histotype (12 cases, 66.6%), followed by nodular melanoma (3; 16.6%), mucous lentiginous melanoma (2; 11.1%) and 1 case of polipoid melanoma (5.5%).
Data on Breslow thickness, presence of ulceration and number of mitosis were available for all but one vulvar melanomas (n=22). Median Breslow thickness for the whole cohort was 5 mm with interquartile range: 3 to 9 mm. The vulvar cohort has a median Breslow thickness of 4.5 mm (interquartile range: 2.3 to 5 mm), significantly (P=0.02) lower than the vaginal cohort (median Breslow: 9 mm, interquartile range: 5 to 15 mm). Breslow thickness was associated with progression free survival and overall survival (P=0.01 and 0.03; adjusting for age and site) (Figure1I). Sixteen out of 22 primary vulvar and vaginal melanomas were ulcerated (72.8%). Median number of mitosis per square mm for the whole cohort of primary vulvar and vaginal melanomas was 11.5 with interquartile range of 6 to 15 (median number: 12 and 10, interquartile range 6-25 and 7-15 for vulvar and vaginal melanomas, respectively).
Surgical treatment of the primary tumor
Patients with primary vulvar melanoma (n=14) received the following treatments: radical vulvectomy (n=5), hemivulvectomy (n=4) and wide local excision (n=5). Patients with a primary vaginal melanoma (n=7) received vaginectomy (n=3), vaginectomy and hysterectomy (n=3) and exenteratio (n=1).The two patients with synchronous vulvar and vaginal melanoma received hemivulvectomy and vaginectomy in one case and hemivulvectomy, vaginectomy and hysterectomy in the other case.
Lymph node status management
Sentinel lymph node biopsy was performed in 9 out 14 patients with primary vulvar melanoma. Three positive patients underwent therapeutic node dissection. Five patients with primary vulvar melanoma had no sentinel lymph node biopsy and 4 of them had an elective lymph node dissection. Three out of these 4 cases had confluent metastatic nodes or more than seven metastatic lymph nodes. One patient refused any further treatment after the excision of the primary vulvar melanoma. Five out of 7 patients with primary vaginal melanoma and 2 cases of concomitant vulvar and vaginal melanoma underwent elective lymph node dissection.
Follow-up events
Median follow-up was 4.1 years. Data on loco-regional recurrence were retrieved for 22 out of 23 patients included in the study. Fourteen patients (63.6%) developed at least one regional event during follow-up. Regional event was defined as melanoma recurrence at the genito-urinary tract or at the regional lymph nodes after radical treatment of primary tumor. Other 4 patients developed distant metastases. In 10 patients loco-regional events preceded the onset of distant metastases. Sixteen out of 23 patients died for the disease, with an overall median survival of 41 months (95%CI: 11-64), and of 45 (95%CI: 11-100) and 7 months (95%CI: 3-30), for vulvar and vaginal melanoma, respectively.
Molecular profile
All cases were screened for KIT alterations and 4 specimens (3 vulvar melanoma and 1 vaginal melanoma) harbored KIT mutation (17%) (Figure1). NRAS alterations were observed in 2 of the 15 available samples (13%), including 1 case of primary vulvar melanoma and 1 case of primary vaginal melanoma. BRAF mutation was detected in 1 vulvar melanoma (5%) among the 19 vulvo-vaginal cases analyzed. KIT mutations were located in exon 11 (codons 560 and 578) and exon 13 (codon 655), while NRAS and BRAF alterations involved exon 2 (codon 61) and exon 15 (codon 600), respectively (Table 1).
In two mutated cases, tissue from recurrence was available, including one loco-regional recurrence and one lymph node metastasis that harbored BRAF and NRAS mutations, respectively. Both specimens maintained the very same mutation detected in the primary tumors.
In our series, mutations of KIT showed a significant association (P=0.03) with patient age at diagnosis. Patients with KIT mutation had significant older age (78 vs. 67 years). No association was found with clinical stage, histology, ulceration or site of onset. KIT mutation was found to be associated with significantly lower number of mitoses (P=0.05, adjusting for age and site; Table 2) and with a significantly lower Breslow thickness (P=0.05 adjusting for age and site; Table 3). BRAF and NRAS mutations were not found to be significantly associated with prognostic factors nor follow-up events.
Table 2. Frequencies of patients by number of mitoses and gene status
| |
|
n. of Mitosis
|
|
| |
|
≤6
|
>6
|
P-value*
|
|
KIT
|
WT†
|
3
|
15
|
0.05
|
| |
|
50%
|
94%
|
|
| |
p.V560D, p.Y578H, p.N655K
|
3
|
1
|
|
| |
|
50%
|
6%
|
|
|
BRAF
|
WT†
|
5
|
12
|
0.99
|
| |
|
83%
|
75%
|
|
| |
p.V600
|
0
|
1
|
|
| |
|
0%
|
6%
|
|
| |
Missing
|
1
|
3
|
|
| |
|
17%
|
19%
|
|
|
NRAS
|
WT†
|
4
|
9
|
0.55
|
| |
|
67%
|
56%
|
|
| |
p.Q61R
|
0
|
1
|
|
| |
|
0%
|
6%
|
|
| |
Missing
|
2
|
6
|
|
| |
|
33%
|
38%
|
|
*P-value from logistic model adjusted for age and site; †WT: wild-type
Table 3. Median and interquartile range of Breslow thickness by gene status
| |
|
N
|
Median Breslow
|
Lower Quartile
|
Upper Quartile
|
P-value*
|
|
KIT
|
WT†
|
19
|
5
|
4
|
12
|
0.05
|
|
p.V560D, p.Y578H, p.N655K
|
4
|
3
|
2
|
3.8
|
|
|
BRAF
|
WT†
|
17
|
5
|
4
|
8
|
0.64
|
|
pV600
|
1
|
2.1
|
|
|
|
|
Missing
|
4
|
7.2
|
1.7
|
12
|
|
|
NRAS
|
WT†
|
13
|
4.5
|
3
|
8
|
0.86
|
|
p.Q61R
|
1
|
9
|
|
|
|
|
Missing
|
8
|
5
|
2
|
8.5
|
|
2021 Copyright OAT. All rights reserv
*P-value from ANCOVA model adjusted for age and sites; †WT: wild-type
Discussion
In this study wwe evaluated the mutational status of three drugable genes (KIT, BRAF, NRAS) in a single centre cohort of genital melanomas. The frequencies of KIT mutations detected in our series were 4/23 (17%) melanomas, 3 arose in vulva and 1 in vagina. Omholt et al. found KIT mutations in 35% of vulvar melanomas; however, no KIT mutations were found in the 7 vaginal melanomas analyzed [21]. Recently, both Aulmann [22] and Rouzbahman [23] described KIT mutations in 18% and 27.6% of vulvar melanomas respectively, but in none of vaginal melanomas tested. Even if KIT mutation appears to be a peculiar sign of vulvar onset, other studies have found mutations even in 1 of 4 [24] and 1 of 24 vaginal melanomas [25]. These differences might be due to the fact that categorizing vulvar versus vaginal melanomas on the basis of the site of onset is demanding and different molecular studies have classified vulvar and vaginal melanomas together as vulvo-vaginal [37], urogenital [38] and genital melanomas [39]. In out cohort, KIT mutated status was associated (6 mitosis cut-off) and lower Breslow’s thickness (5 mm cut-off). Since a greater Breslow thickness was associated with a significantly worse prognosis (Figure1I), we could speculate that KIT mutated melanomas might have a better prognosis than KIT wild-type cases. However, due to the small sample size, we did not find any statistically significant evidence of a link between KIT mutational status and other clinical parameters, such as lymph node status, distant metastases occurrence and overall survival, according to the findings of Omholt el al. [21].
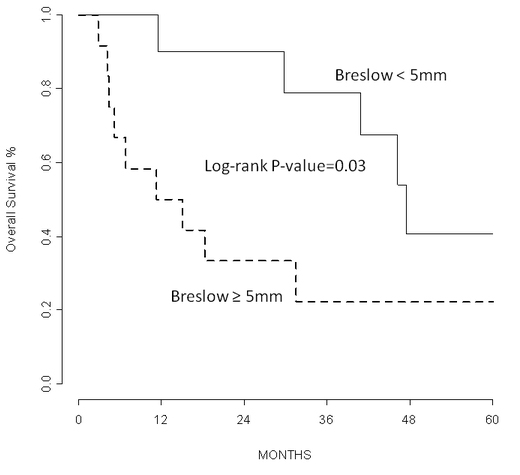
Figure 2. Kaplan-Meier curves show that Breslow thickness was associated with overall survival (P= 0.03; adjusting for age and site).
Different studies have reported on the presence of NRAS mutation in vaginal melanomas with a wide frequency range: 2/15 (13.3%) [22], 3/7 (42.9%) [21], 3/14 (21.4%) [25]. However, these data lead to a significantly higher proportion of NRAS mutated vaginal melanomas than mucosal melanomas of other sites [21]. In our cohort, NRAS mutation was detected in one primary vaginal melanoma specimen and in one vaginal recurrence of a primary vulvar melanoma, and accounts for 13% (2/15) of the all cases. Moreover, we found only one BRAF mutation (5%), according to the studies describing few BRAF mutations in genital lesions [40].
To the best of our knowledge, this is the first report of the presence of genes mutations in primary vulvo-vaginal melanomas that were maintained in the subsequent metastatic or recurrence site. These data were already reported for other site of onset. Although detected on a limited number of patients, these findings highlighted the importance of these genes as neoplastic progression drivers in this subset of melanomas and possible drugable targets.
The cumulative frequency of oncogenic mutations in melanomas of the female genital reported in our series corresponds to the one that has been proposed by Tseng et al. [41]. Although, other studies have showed a lower frequency of mutations, mainly of KIT gene [42], these discrepancies might be related to the small number of cases analyzed and to the different analytical assays used.
However, regardless the actual frequency of these mutations, the presence of BRAF, KIT and NRAS gene aberrations could be targeted by specific pharmacological inhibitors. Several studies have showed that the molecular characterization allowed the selection of patients who could benefit from target therapies. The type of KIT mutation and the ratio of mutant to wild-type KIT alleles were predictive of clinical response to imatinib [27,28]. Moreover other drugs such as dasatinib [29] and sunitinib [30] and nilotinib [31] are under investigation in phase II clinical trials for KIT mutated patients. The clinical relevance of BRAF inhibitors in treatment of melanoma patients carrying BRAF codon 600 mutations is well known [32,33] and new studies highlight the importance of a combination with MEK inhibitors [34]. Ascierto et al. reported that binimetinib have shown a robust activity specifically in NRAS mutated melanoma [35] and new clinical trials are enrolling NRAS mutated patients [36]. Unfortunately, no molecular target therapy was administered to any patients investigated in this study and no further data on treatment response and survival are available.
Therefore, since vulvo-vaginal melanoma could harbor KIT, NRAS, BRAF mutations (overall 30% in our study), which are also maintained in the recurrent/metastatic disease, molecular analyses need to guide current therapeutic choices for these patients. However, further trials and multicenter studies are required to better understand the clinical impact of target therapy in urogenital melanomas.
Autorship
Conception and design: Caterina Fumagalli, Massimo Barberis, Giovanni Mazzarol; Acquisition of data: Caterina Fumagalli, Giulio Tosti, Sara Gandini, Davide Vacirca, Marco Turina, Giovanni Mazzarol; Analysis and interpretation of data: Caterina Fumagalli, Giulio Tosti, Sara Gandini, Davide Vacirca, Marco Turina, Elena Guerini-Rocco, Massimo Barberis, Giovanni Mazzarol; Writing, review, and/or revision of the manuscript: Caterina Fumagalli, Giulio Tosti, Sara Gandini, Davide Vacirca, Marco Turina, Elena Guerini-Rocco, Massimo Barberis, Giovanni Mazzarol.
Acknowledgment
Laboratory technicians of division of pathology, European Institute of Milan.
References
- Ragnarsson-Olding B, Johansson H, Rutqvist LE, Ringborg U (1993) Malignant melanoma of the vulva and vagina. Trends in incidence, age distribution, and long-term survival among 245 consecutive cases in Sweden 1960-1984. Cancer 71: 1893-1897. [Crossref]
- Weinstock MA1 (1994) Malignant melanoma of the vulva and vagina in the United States: patterns of incidence and population-based estimates of survival. Am J Obstet Gynecol 171: 1225-1230. [Crossref]
- Stang A, Streller B, Eisinger B, Jackel KH (2005) Population-based incidence rates of malignant melanoma of the vulva in Germany. Gynecol Oncol 96: 216-221. [Crossref]
- Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V (2012) Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol 5: 739-753. [Crossref]
- Piura B1 (2008) Management of primary melanoma of the female urogenital tract. Lancet Oncol 9: 973-981. [Crossref]
- Hu DN, Yu GP, McCormick SA (2010) Population-based incidence of vulvar and vaginal melanoma in various races and ethnic groups with comparisons to other site-specific melanomas. Melanoma Res 20: 153-158. [Crossref]
- Chang AE, Karnell LH, Menck HR (1998) The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 83: 1664-1678. [Crossref]
- Ragnarsson-Olding BK, Nilsson BR, Kanter-Lewensohn LR, Lagerlaf B, Ringborg UK (1999) Malignant melanoma of the vulva in a nationwide, 25-year study of 219 Swedish females: predictors of survival. Cancer 86: 1273-1284. [Crossref]
- Sugiyama VE, Chan JK, Shin JY, Berek JS, Osann K, et al. (2007) Vulvar melanoma: a multivariable analysis of 644 patients. Obstet Gynecol 110: 296-301. [Crossref]
- Lotem M, Anteby S, Peretz T, Ingber A, Avinoach I, et al. (2003) Mucosal melanoma of the female genital tract is a multifocal disorder. Gynecol Oncol 88: 45-50. [Crossref]
- Tcheung WJ, Selim MA, Herndon JE 2nd, Abernethy AP, Nelson KC (2012) Clinicopathologic study of 85 cases of melanoma of the female genitalia. J Am Acad Dermatol 67: 598-605. [Crossref]
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, et al. (2012) Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline--Update 2012. Eur J Cancer 48: 2375-2390. [Crossref]
- Kaufman HL, Kirkwood JM, Hodi FS, Agarwala S, Amatruda T, et al. (2013) The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol 10: 588-598. [Crossref]
- Seifried S, Haydu LE, Quinn MJ, Scolyer RA, Stretch JR, et al. (2015) Melanoma of the vulva and vagina: principles of staging and their relevance to management based on a clinicopathologic analysis of 85 cases. Ann Surg Oncol 22: 1959-1966. [Crossref]
- Frumovitz M, Etchepareborda M, Sun CC, Soliman PT, Eifel PJ, et al. (2010) Primary malignant melanoma of the vagina. Obstet Gynecol 116: 1358-1365. [Crossref]
- Phillips GL, Bundy BN, Okagaki T, Kucera PR, Stehman FB (1994) Malignant melanoma of the vulva treated by radical hemivulvectomy. A prospective study of the Gynecologic Oncology Group. Cancer 73: 2626-2632. [Crossref]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, et al. (2005) Distinct sets of genetic alterations in melanoma. NEJM 353: 2135-2147. [Crossref]
- Baiter M, Schuler G, Hartmann A, Schneider-Stock R, Heinzerling L (2015) Pathogenetic Implications of BRAF Mutation Distribution in Stage IV Melanoma Patients. Dermatology 231: 127-133. [Crossref]
- Curtin JA, Busam K, Pinkel D, Bastian BC (2006) Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 24: 4340-4346. [Crossref]
- Zebary A, Jangard M, Omholt K, Ragnarsson-Olding B, Hansson J (2013) KIT, NRAS and BRAF mutations in sinonasal mucosal melanoma: a study of 56 cases. Br J Cancer 109: 559-564. [Crossref]
- Omholt K, Grafstram E, Kanter-Lewensohn L, Hansson J, Ragnarsson-Olding BK (2011) KIT pathway alterations in mucosal melanomas of the vulva and other sites. Clin Cancer Res 17: 3933-3942. [Crossref]
- Aulmann S, Sinn HP, Penzel R, Gilks CB, Schott S, et al. (2014) Comparison of molecular abnormalities in vulvar and vaginal melanomas. Mod Pathol 27: 1386-1393. [Crossref]
- Rouzbahman M, Kamel-Reid S, Al Habeeb A, Butler M, Dodge J, et al. (2015) Malignant Melanoma of Vulva and Vagina: A Histomorphological Review and Mutation Analysis--A Single-Center Study. J Low Genit Tract Dis 19: 350-353. [Crossref]
- Torres-Cabala CA, Wang WL, Trent J, Yang D, Chen S, et al. (2009) Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol 22: 1446-1456. [Crossref]
- van Engen-van Grunsven AC, Kasters-Vandevelde HV, De Hullu J, van Duijn LM4, Rijntjes J, et al. (2014) NRAS mutations are more prevalent than KIT mutations in melanoma of the female urogenital tract--a study of 24 cases from the Netherlands. Gynecol Oncol 134: 10-14. [Crossref]
- Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, et al. (2013) Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 31: 3182-3190. [Crossref]
- Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, et al. (2011) KIT as a therapeutic target in metastatic melanoma. JAMA 305: 2327-2334. [Crossref]
- Guo J, Si L, Kong Y, Flaherty KT, Xu X, et al. (2011) Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 29: 2904-2909. [Crossref]
- Kluger HM, Dudek AZ, McCann C, Ritacco J, Southard N, et al. (2011) A phase 2 trial of dasatinib in advanced melanoma. Cancer 117: 2202-2208. [Crossref]
- Minor DR, Kashani-Sabet M, Garrido M, O'Day SJ, Hamid O, et al. (2012) Sunitinib therapy for melanoma patients with KIT mutations. Clin Cancer Res 18: 1457-1463. [Crossref]
- Cho JH, Kim KM, Kwon M, Kim JH, Lee J (2012) Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs 30: 2008-2014. [Crossref]
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, et al. (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380: 358-365. [Crossref]
- Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, et al. (2012) Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. NEJM 366: 707-714. [Crossref]
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, et al. (2014) Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. NEJM 371: 1877-1888. [Crossref]
- Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, et al. (2013) MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 14: 249-256. [Crossref]
- Zimmer L, Barlesi F, Martinez-Garcia M, Dieras V, Schellens JH, et al. (2014) Phase I expansion and pharmacodynamic study of the oral MEK inhibitor RO4987655 (CH4987655) in selected patients with advanced cancer with RAS-RAF mutations. Clin Cancer Res 20: 4251-4261. [Crossref]
- Schoenewolf NL, Bull C, Belloni B, Holzmann D, Tonolla S, et al. (2012) Sinonasal, genital and acrolentiginous melanomas show distinct characteristics of KIT expression and mutations. Eur J Cancer 48: 1842-1852. [Crossref]
- Abysheva SN, Iyevleva AG, Efimova NV, Mokhina YB, Sabirova FA, et al. (2011) KIT mutations in Russian patients with mucosal melanoma. Melanoma Res 21: 555-559. [Crossref]
- Satzger I, Schaefer T, Kuettler U, Broecker V, Voelker B, et al. (2008) Analysis of c-KIT expression and KIT gene mutation in human mucosal melanomas. Br J Cancer 99: 2065-2069. [Crossref]
- Wong CW, Fan YS, Chan TL, Chan AS, Ho LC, et al. (2005) BRAF and NRAS mutations are uncommon in melanomas arising in diverse internal organs. J Clin Pathol 58: 640-644. [Crossref]
- Tseng D, Kim J, Warrick A, Nelson D, Pukay M, et al. (2014) Oncogenic mutations in melanomas and benign melanocytic nevi of the female genital tract. J Am Acad Dermatol 71: 229-236. [Crossref]
- Pappa KI, Vlachos GD, Roubelakis M, Vlachos DE, Kalafati TG, et al. (2015) Low mutational burden of eight genes involved in the MAPK/ERK, PI3K/AKT, and GNAQ/11 pathways in female genital tract primary melanomas. Biomed Res Int: 303791. [Crossref]


