Abstract
Late recurrences of melanoma are well known but uncommon. We present a case of a 78-year-old Caucasian female with a history of melanoma involving the medial right ankle initially diagnosed in 1988 and excised with negative margins (additional details are not known as the primary specimen and pathology report were not available for review). In 2004 (16 years after initial diagnosis), the patient noted a new nodule at the same site of the previous melanoma excision; this was biopsied and showed fibrosis but no melanoma. The nodule continued to enlarge and in 2006 a repeat biopsy showed recurrent melanoma. After excision of this recurrence, the patient developed 5 additional subsequent recurrences in the same location over the next 8 years. The tumor cells expressed S100 protein and SOX-10 by immunohistochemistry but were negative for Melan A and HMB-45. Fluorescent in-situ hybridization was negative for EWSR1 gene rearrangement but positive for BRAF V600E mutation, both findings supporting a diagnosis of melanoma and arguing against clear cell sarcoma. Despite the multiple local recurrences, no obvious regional or distant metastases had been identified clinically; in 2012 a full body PET/CT was negative. However, the patient presented with yet another local recurrence in 2014, and a repeat PET/CT performed at that time revealed three pulmonary nodules and one inguinal nodule presumed to represent distant metastases. Courses of vemurafenib and ipilimumab were both attempted (separately), but for both regimens the patient discontinued treatment after only one dose due to side effects. She was referred to hospice in late 2014. In early 2016, she returned to the surgeon’s office, feeling well and having outlived her hospice referral; her only complaint was a new recurrent nodule on her ankle that she wished to have excised. The excision specimen again showed recurrent melanoma. Between 1988 and 2016, a period of 28 years after the initial diagnosis of melanoma of the right ankle, the patient’s tumor had recurred locally 7 times with no definitive evidence of regional or distant metastasis (the probable lung metastases seen on 2014 PET/CT have not yet been re-evaluated). The extremely unusual pattern of recurrences in this case highlights the importance of long term or even life-long clinical follow-up in treating patients with melanoma and demonstrates the unusual length of disease-free survival that can precede disease recurrence.
Key words
melanoma, local recurrence, late recurrence, BRAF, clear cell sarcoma
Introduction
Late recurrences, defined as more than 10 or 15 years after primary diagnosis of melanoma, are well known but uncommon and often present with distant recurrence/metastasis or regional metastasis [1]. True local recurrences of melanoma following complete excision with clear margins are uncommon, typically occur within 15 months after primary diagnosis, and are often associated with high mortality rates following recurrence [2]. However, multiple late local recurrences without further disease progression are exceedingly rare [3].
Case report
We present a case of a 78-year-old Caucasian female with melanoma of the right medial ankle initially diagnosed in 1988 (at the age of 50 years) and excised with negative margins (additional details are not known as the specimen and pathology report were not available for review). In 2004, the patient noted a new nodule at the site of her previous surgery. Biopsy of this lesion revealed fibrosis but no melanoma was identified. However, the lesion continued to enlarge and, in 2006, an additional biopsy revealed a dermal nodule of melanoma consistent with recurrent/metastatic growth pattern (Figures 1A and 1B). This was subsequently excised and was found to express S100 protein, but it did not express Melan-A or HMB-45 by immunohistochemistry. The tumor was composed of sheets of large epithelioid melanocytes with nuclear atypia and abundant eosinophilic cytoplasm. Some cells displayed rhabdoid cytologic features. Zones of tumor necrosis were identified. The lesion was within 0.8 mm of the margin; an additional re-excision was not performed at that time. In 2008, a new nodule arose at the previous surgical site; excision again showed melanoma with a recurrent/metastatic growth pattern and prominent necrosis (margins were negative) (Figure 2). In 2009, another nodule arose at this site; wide local excision again revealed melanoma in the dermis with negative surgical margins. In 2010, another nodule of recurrent melanoma in the dermis was excised, this time with positive deep margin (Figure 3). Although the histologic features were not suggestive of it, an alternative diagnosis of clear cell sarcoma of soft tissue was considered in light of the distal extremity site, absence of pathology data from the initial melanoma diagnosis, and the very unusual pattern of multiple local recurrences. However, fluorescent in-situ hybridization (FISH) was negative for the EWSR1-ATF1 gene rearrangement that is characteristic of clear cell sarcoma. In 2012, the patient again presented with a 1 cm mass on the right ankle at the location of the previous excisions; this was diagnosed as melanoma by fine needle aspiration. At this time, a PET/CT scan was performed and showed only positive uptake in the ankle area of known recurrence but no regional or distant metastatic disease. A wide local excision of this revealed a 1.5 cm nodule of recurrent melanoma involving soft tissue with lymphovascular invasion and focal involvement of the margin. Immunostains were performed on this 2012 recurrence: the tumor expressed S100 protein and SOX-10 but was negative for Melan-A and HMB-45. Perineural invasion was not identified in this or any of the earlier recurrence specimens. In 2014, recurrent melanoma was again excised from the ankle. This time the tumor displayed spindled and desmoplastic features, a pattern not seen in previous specimens; margins were positive. Mutational analysis on the 2014 specimen revealed a BRAF V600E mutation. A PET/CT scan performed shortly after this excision revealed likely progression to stage IV disease with 3 new hypometabolic pulmonary nodules and a nonspecific right inguinal nodule. Courses of vemurafenib and ipilimumab were both attempted, but she experienced severe side effects on both regimens and voluntarily discontinued each after only one dose. In late 2014, the patient was referred to hospice care. Amazingly, the patient returned to clinic in 2016 (shortly before submission of this manuscript). She had outlived her hospice referral and was asymptomatic aside from a new recurrent nodule on her ankle that she wished to have excised (Figure 4). Palliative re-excision of this new nodule was performed and showed multifocal involvement by recurrent melanoma with positive deep margin. Lymphovascular invasion was present, but perineural invasion was not identified. No desmoplastic or spindle cell melanoma components were seen. No new imaging studies have yet been performed to evaluate the inguinal or lung nodules that were noted on PET/CT in 2014. Figure 5 illustrates the convoluted timeline of clinical events for this patient.
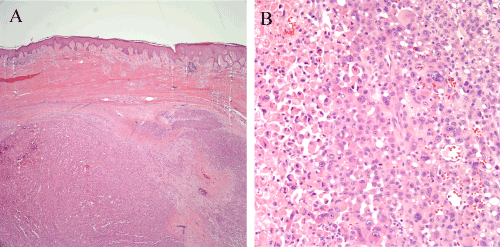
Figure 1. Recurrent melanoma of the right ankle excised in 2006, 18 years after initial excision of a primary melanoma from that same site. (1A) A large nodule of recurrent melanoma fills the dermis. Dense fibrosis consistent with mature scar is present between the nodule and the overlying epidermis. (1B) Sheets of large atypical epithelioid melanocytes comprise the tumor. Some of the cells have rhabdoid cytologic features. A zone of tumor necrosis is visible at the left side of the image.
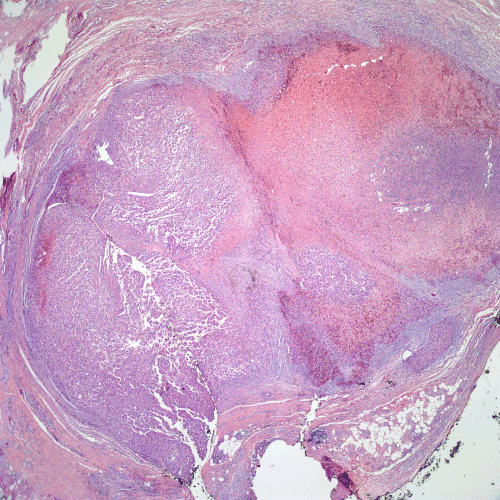
Figure 2. Recurrent melanoma excised in 2008. A large nodule of melanoma with a broad zone of tumor necrosis is present in the deep dermis.
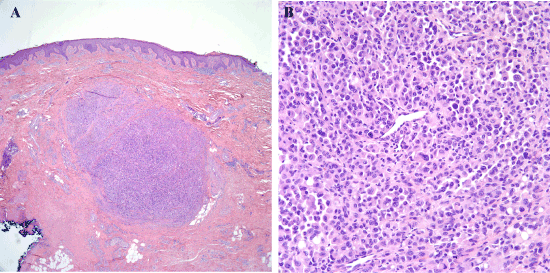
Figure 3. Recurrent melanoma excised in 2010. (3A) As with previous excisions, there is a nodule of melanoma within the fibrotic dermis. (3B) Sheets of malignant melanocytes comprise the nodule, and many of these have plasmacytoid or rhabdoid cytologic features.
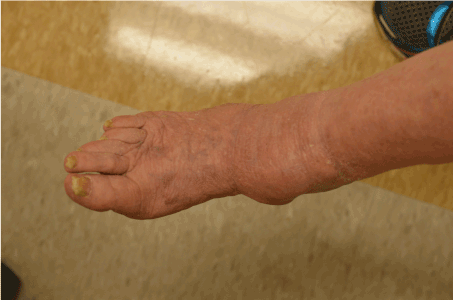
Figure 4. Clinical image of 2016 recurrent melanoma. There is a nodule on the medial right ankle. The adjacent skin shows scarring from the multiple previous surgical procedures. Image courtesy of Angela Cheng, M.D.
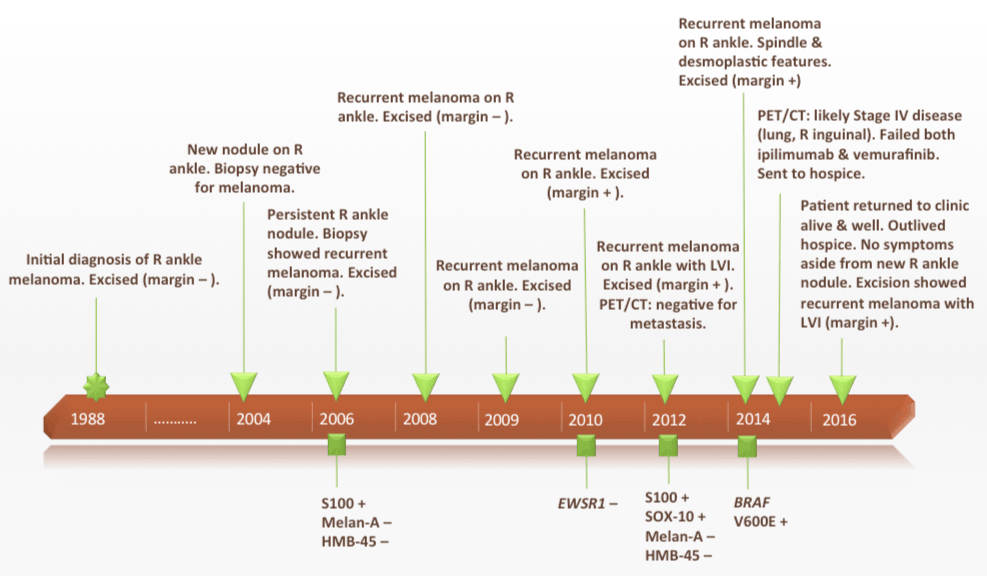
Figure 5. A timeline showing the patient’s primary melanoma and multiple subsequent recurrences over a 28-year period (1988-2016).
Review of the literature
Case reports and literature reviews dating from 1931 to present on late and local melanoma recurrences were reviewed using the Texas Medical Center online library search engine. The criterion for late recurrence in this paper is at least 10 years after initial diagnosis. Local recurrences are considered as those arising in or immediately around the scar or site of original diagnosis without evidence of regional or distant disease. Reports of late local recurrences with simultaneous regional or distant metastatic disease were excluded. Age, gender, location, Clark level, Breslow thickness, disease free interval, and final outcome were recorded when available.
Eighteen late local recurrences without evidence of concurrent disease were documented in the literature. Late local recurrences were most common on the lower extremities (9 cases), followed by head/neck (5 cases), trunk (2 cases), and upper extremity (1 case). There was striking female predominance (16 out of 18 patients were female). The average age at primary diagnosis was 48 years. The average disease free interval was 14.4 years, with a range of 10-22.8 years. Only six cases reported Clark level and Breslow thickness. Three cases were Clark level II, one was Clark level III, and one was Clark level IV. The case reported by Thomas did not have a Clark level in the initial diagnosis, but the two recurrent cases were Clark level II and IV, respectively. The Breslow thickness averaged 1.18 mm (range 0.4-1.85 mm). At follow up of varying lengths (range 5-216 months excluding the Otsu paper), five patients died of disease, four were alive with no evidence of disease, four were only reported as alive with no comment regarding disease status, three statuses were unknown, one experienced two local recurrences, and one had a regional and systemic disease in four years time but was still being treated at the time of reporting [3-11].
Discussion
It is estimated that 0.98-6.7% of melanomas recur after greater than 10 years of disease free survival [1,6,8]. It is reported that the recurrence rate after surviving at least 15 years disease free is 2.0% [1]. In these statistics, the term “recurrence” may indicate local recurrence, regional metastasis, and/or distant metastasis or all of these. The incidence of local recurrence is estimated to be 3-5% in some studies [2,12,13]. and these recurrences usually present within 5 years after diagnosis of the primary melanoma [14]. However, the incidence of late local recurrences (at the site of the primary tumor but in the absence of regional or distant metastasis) has not been clearly reported, as local recurrence data are sometimes grouped with regional recurrence data in the literature.
Our patient had 3 local recurrences with clear margins of excision and 4 subsequent local recurrences with margin involvement. Lung and inguinal nodules were seen on the PET/CT in 2014 and were favored to represent metastases at that time. However, the patient is alive and well currently, nearly 2 years after these probable metastases were identified, and has no clinical suggestion of further disease progression despite receiving no additional systemic therapy since 2014. This suggests that these nodules may have been false positive findings on the 2014 PET/CT. However, as no scan or biopsy has yet been performed to further evaluate these lung or inguinal nodules, we cannot yet be certain of this. Aside from this unusual caveat, this patient had no other evidence or suggestion of regional or distant metastasis over the entire 28-year period since her primary diagnosis in 1988, despite numerous local recurrences at the primary site during that time period. This pattern of multiple late local recurrences without distant or regional metastasis is extremely unusual and we were only able to identify one similar case in the literature [3].
Review of the literature on late local recurrences revealed only 18 cases that fit the criteria set above (Table 1) [1,4-11]. However, unlike the current case, none of these patients experienced local recurrences in the same site more than once. Studies and reviews of local recurrence by Urist, Cohn-Cedermark, Dong, and Heenan did not include late recurrences [2,13,15,16]. Another study by Crowley, et al, reported 18 patients with local recurrence after at least 10 years of disease free interval, but the follow up information on local recurrence was grouped with regional node and in-transit recurrence and thus this data was excluded from our review [17]. Several other reports in the literature included late recurrences, but their cases were not exclusively local by our definition and/or included concurrent disease [18-26].
Table 1. Current Case and Literature Review of Patients with Late Local Recurrence of Melanoma
Source |
Age, Gender |
Location |
DFI
(yr) |
Clark level/
Breslow thickness (mm) |
Outcome* |
Tahery |
47, F |
Left calf |
18 |
II/0.9 |
DOD 23 mo |
McEwan |
39, F |
Face |
18.2 |
II/0.4 |
NED 5 mo |
Callaway |
39, F |
Left shin |
15.3 |
II/0.2 |
DOD 18 mo |
Avril |
33, F |
Leg |
15 |
NA |
NED 60 mo |
|
53, F |
Leg |
22.8 |
IV/>1.85 |
NED 216 mo |
Tsao |
40, M |
Right abdomen |
20 |
III/0.95 |
NED 24 mo |
Briele |
38, F |
Right leg |
11 |
III/1.45 |
Alive with disease: recurrence 48 mo, regional and systemic |
Wilbur |
49, F |
Cheek |
12 |
NA |
Died |
Olson |
49, F |
Hand |
11 |
NA |
Died |
Otsu |
49, M |
Foot |
10 |
NA |
Alive |
|
56, F |
Head/neck |
11 |
NA |
Alive |
|
54, F |
Foot |
13 |
NA |
Alive |
|
63, F |
Leg |
14 |
NA |
Alive |
|
67, F |
Head/neck |
11 |
NA |
Alive |
|
47, F |
Leg |
13 |
NA |
Alive |
|
45, F |
Trunk |
12 |
NA |
Died |
|
46, F |
Head/neck |
13 |
NA |
Alive |
Tomas |
50, F |
Left forearm |
19, 8 |
II/0.2, IV/2.2 |
Alive |
Current case |
50, F |
Right ankle |
18, 2, 1, 1, 2, 2, 2 |
NA |
Alive with disease |
Legend: DFI= disease free interval; NA = not available. *Outcomes are given as follows: DOD= died of disease, NED= no evidence of disease, outcome is given as status at time of each report with months calculated from date of recurrence to date of last clinical follow up. Reports for which no specific details were given as to cause of death, the outcome is listed as ‘Died’. Reports for which the disease status was not given, the outcome is listed as “Alive’.
A case reported by Tomas of a 50-year-old female with a superficial spreading melanoma on the upper extremity that recurred 19 years later in the excision scar and again eight years later. The second recurrence was similar to our case in that only a dermal deposit of melanoma with no connection to the epidermis was present. Small deposits of melanoma discontinuous from the main lesion were also seen, and there was also extensive perineural invasion. The patient did not have evidence of distant metastasis.
Local recurrence is associated with better overall survival than other types of recurrences (i.e. – regional or distant) provided the local recurrence is not associated with regional or distant metastasis [17-19]. Several studies concur that a longer disease free interval is a predictor of superior survival for patients with local recurrence [15,17,19,27]. The reported five year survival of patients with local recurrence varies from 34% [13] up to 80% [1]; although prognostic data of late local recurrence has not been separated out from overall local recurrence in prior studies, our review suggests that the survival statistics may be similar for both groups. Including our case, final outcome was reported in 16 patients with late local recurrence. Time from recurrence to follow up was not recorded for all of these cases, but 11 (68.7%) were alive at follow up, and ten (62.5%) had no evidence of further disease. However, longer follow up is necessary to draw definitive conclusions on prognostic information, especially in this small subset of patients who have already demonstrated the potential for long apparently disease-free intervals prior to recurrence.
Anatomic location has also been of importance in predicting whether melanomas are likely to recur. Tsao, et al., found that approximately 39% of reported cases of “ultra-late” recurrence were lower extremity melanomas with regional recurrence [1]. This pattern was noted in our review of the literature and in the current case: 10/19 (52.6%; our case included) late local recurrences were in the lower extremity. Observations of Tsao, Briele, Raderman, and Levy et al. found that the overwhelming majority of cases of late recurrence occurred in females, which was also reflected in our review (16/18, 89% or 17/19, 89.5% including our case) [1,8,28,29]. However, Tsao, et al. previously addressed the issues of whether a selection bias exists due to the increased survival in general of women with melanoma [1]. It would appear from the data in the current study that late local recurrence without regional spread of tumor is also more frequently found in females and tends to occur on the lower extremity. One of many possible explanations is that recurrence in other anatomical sites tends to drain to lymphatics at a faster rate due to closer proximity to the nodal basin as compared to the lower extremity where the nodal basin is further away from the primary site.
It is important to consider the diagnosis of clear cell sarcoma (formerly known as “melanoma of soft parts”) in the differential diagnosis of melanoma in the distal extremity presenting with a deep nodular growth pattern without an associated in-situ component or connection to the epidermis. Clear cell sarcoma is typically composed of monotonous (rather than pleomorphic) cells with pale eosinophilic or clear cytoplasm arranged in fascicles and elongated nests invested in dense fibrous tissue. The tumors are essentially identical to melanoma in their immunophenotype as they express markers of melanocytic differentiation by immunohistochemistry including S100 protein, SOX-10, Melan-A (MART-1), and HMB-45. However, they are characterized by the characteristic molecular signature t(12;22)(q13;q12), a translocation which corresponds to the EWSR1-ATF1 gene fusion. Fluorescent in-situ hybridization (FISH) utilizing an EWSR1 breakapart probe can demonstrate the presence of this translocation and confirm the diagnosis of clear cell sarcoma [30,31]. Clear cell sarcoma also differs from melanoma in that it often has a more indolent, protracted clinical course with late local recurrence and/or late regional or distant metastases.
Further evaluation of melanomas with late local recurrences for unique biologic factors or specific mutations may provide insight into their indolent behavior as compared to typical melanomas. Genetic testing of melanomas is becoming more relevant as various targeted treatments for patients with metastatic melanoma are emerging. However, testing of patients with only local recurrences is not common, and there is much progress to be made in identifying additional risk factors and indications for genetic testing. Nevertheless, our report demonstrates the variance in behavior that melanoma can exhibit and provides reason to further stratify melanomas based on their patterns of behavior as yet another step to increase our understanding of the tumor biology of melanoma. It also emphasizes that high-risk histologic features do not always correlate with rapid aggressive clinical progression of disease. Lastly, the current case and review of the literature reinforce the recommendation that physicians continue to carefully screen melanoma patients for regional and distant metastatic disease as well as local recurrence for the life of the patient.
References
- Tsao H, Cosimi AB, Sober AJ (1997) Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer 79: 2361-2370. [Crossref]
- Cohn-Cedermark G, Månsson-Brahme E, Rutqvist LE, Larsson O, Singnomklao T, et al. (1997) Outcomes of patients with local recurrence of cutaneous malignant melanoma: a population-based study. Cancer 80: 1418-1425. [Crossref]
- Thomas S (2007) Recurring Melanoma: A case study. Aust Fam Physician 36: 1015-1017. [Crossref]
- Tahery DP, Moy RL (1992) Lack of predictive factors in late recurrence of stage I melanoma. Int J Dermatol 31: 629-631. [Crossref]
- McEwan L, Smith JG, Matthews JP (1990) Late recurrence of localized cutaneous melanoma: its influence on follow-up policy. Plast Reconstr Surg 86: 527-534. [Crossref]
- Callaway MP, Briggs JC (1989) The incidence of late recurrence (greater than 10 years); an analysis of 536 consecutive cases of cutaneous melanoma. Br J Plast Surg 42: 46-49. [Crossref]
- Avril MF, Nguyen T, Duvillard P, Bognel C, Margulis A (1994) [Late recurrence of melanoma beyond 10 years]. Ann Dermatol Venereol 121: 454-458. [Crossref]
- Briele HA, Beattie CW, Ronan SG, Chaudhuri PK, Das Gupta TK (1983) Late recurrence of cutaneous melanoma. Arch Surg 118: 800-803. [Crossref]
- Wilbur DL, Harman HR (1931) Malignant melanoma with delayed metastatic growths. Annals of Internal Medicine 5: 201-211.
- Olsen G (1966) The malignant melanoma of the skin. New theories based on a study of 500 cases. Acta Chir Scand Suppl 365: 1-222. [Crossref]
- Otsu U, Fukui N, Iki M, Moriwaki S, Kiyokane K (2009) Case of cutaneous malignant melanoma surviving 16 years with late recurrence. J Dermatol 36: 598-603. [Crossref]
- Cruse CW, Wells KE, Schroer KR, Reintgen DS (1992) Etiology and prognosis of local recurrence in malignant melanoma of the skin. Ann Plast Surg 28: 26-28. [Crossref]
2021 Copyright OAT. All rights reserv
- Urist MM, Balch CM, Soong S, Shaw HM, Milton GW, et al. (1985) The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer 55: 1398-1402. [Crossref]
- Griffiths RW, Briggs JC (1986) Incidence of locally metastatic (“recurrent”) cutaneous malignant melanoma following conventional wide margin excisional surgery for invasive clinical stage I tumours: Importance of maximal primary tumour thickness. Br J Surg 73: 349-353. [Crossref]
- Dong XD, Tyler D, Johnson JL, DeMatos P, Seigler HF (2000) Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer 88: 1063-1071. [Crossref]
- Heenan PJ, English DR, Holman CD, Armstrong BK (1992) The effects of surgical treatment on survival and local recurrence of cutaneous malignant melanoma. Cancer 69: 421-426. [Crossref]
- Crowley NJ, Seigler HF (1990) Late recurrence of malignant melanoma. Analysis of 168 patients. Ann Surg 212: 173-177. [Crossref]
- JACKSON R (1965) Observations on the natural course of skin cancer. Can Med Assoc J 92: 564-570. [Crossref]
- Shaw HM, Beattie CW, McCarthy WH, Milton GW (1985) Late relapse from cutaneous stage I malignant melanoma. Arch Surg 120: 1155-1159. [Crossref]
- Schmid-Wendtner MH, Baumert J, Schmidt M, Konz B, Hölzel D, et al. (2000) Late metastases of cutaneous melanoma: an analysis of 31 patients. J Am Acad Dermatol 43: 605-609. [Crossref]
- Ariel IM (1980) Theories regarding the cause of malignant melanoma. Surg Gynecol Obstet 150: 907-917. [Crossref]
- White LP (1963) The role of natural resistance in the prognosis of human melanoma. Ann N Y Acad Sci 100: 115-122. [Crossref]
- Koh HK, Sober AJ, Fitzpatrick TB (1984) Late recurrence (beyond ten years) of cutaneous malignant melanoma. Report of two cases and a review of the literature. JAMA 251: 1859-1862. [Crossref]
- Soong SJ, Harrison RA, McCarthy WH, Urist MM, Balch CM, et al. (1998) Factors Affecting Survival Following Local, Regional, or Distant Recurrence From Localized Melanoma. J Surg Oncol 67: 228-233. [Crossref]
- Roses DF, Harris MN, Rigel D, Carrey Z, Friedman R, et al. (1983) Local and In-Transit Metastases Following Definitive Excision for Primary Cutaneous Malignant Melanoma. Ann Surg 198: 65-69. [Crossref]
- Hansel G, Schönlebe J, Haroske G, Wollina U (2010) Late recurrence (10 years or more) of malignant melanoma in southeast Germany (Saxony): A single-centre analysis of 1881 patients with a follow-up of 10 years or more. J Euro Acad Dermatol Venereol 24: 833-836. [Crossref]
- Karakousis CP, Balch CM, Urist MM, Ross MM, Smith TJ, et al. (1996) Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Ann Surg Oncol 3: 446-452. [Crossref]
- Raderman D, Giler S, Rothem A, Ben-Bassat M (1986) Late metastases (beyond ten years) of cutaneous malignant melanoma. Literature review and case report. J Am Acad Dermatol 15: 374-378. [Crossref]
- Levy E, Silverman MK, Vossaert KA, Kopf AW, Bart RS, et al. (1991) Late recurrence of malignant melanoma: a report of five cases, a review of the literature and a study of associated factors. Melanoma Res 1: 63-67. [Crossref]
- Weiss SW, Goldblum JR, Folpe AL (2008) Enzinger and Weiss’s Soft Tissue Tumors. Mosby Pp. 926-934.
- Zucman J, Delattre O, Desmaze C, Epstein AL, Stenman G, et al. (1993) EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat Genet 4: 341-345. [Crossref]





