Abstract
Squamous cell carcinoma (SCC) develops from keratinocytes. As per the statistical analysis, it is the second most frequent skin cancer after Basal cell carcinoma among non-melanoma skin cancers and its incidences are more than death cases . Australia has the highest incidence as compared to the rest of the world. This type of cancer is caused primarily by the exposure to the UV radiation, coming from the sun. SCC develops due to the alteration in the DNA of the squamous cells present in the outer skin layer. In this carcinoma, an early stage shows replacement of normal epithelial cells by atypical squamous cells throughout the epidermis, resulting in abnormal growth. Immunotherapy has shown to be a promising development in the past few years. The recent activities have increased our understanding of the tumour microenvironment, various immunotherapeutic modalities or combination therapy (like chemotherapy with immunotherapy). The complete perspective of the immunotherapy treatment has not been realized yet. Additionally, the effects of such modalities in combination with immunotherapy in cancer patients are still exploratory phase.
Key words
squamous cell carcinoma (SCC), keratinocytes, basal cell carcinoma, non-melanoma skin cancers, replacement of normal epithelial cells, atypical squamous cells, abnormal growth, chemical carcinogens, human papilloma virus infectio, immune deficient patient, mutation and hypermethylation, tumor suppressor gene (TSG), matrix metalloproteinases (MMP), tumour microenvironment
Abbreviations
EGFR: Epidermal growth factor receptor; HPV: Human papilloma virus infection; MMP: Matrix metalloproteinases; MABs: Monoclonal antibodies; SCC: Squamous cell carcinoma; TSG: Tumor suppressor gene
Introduction and epidemiology
Squamous cell carcinoma (SCC) develops from keratinocytes [1]. According to the Skin cancer foundation, in 2012, around 700,000 new cases of sqamous cell carcinoma were diagnosed in the U.S and in the same year, 3900- 8800 cases of death were reported [2]. As per the statistical analysis, it is the second most frequent skin cancer after Basal cell carcinoma among non-melanoma skin cancers [2]. Incidence of this type of skin cancer has increased up to 200% in the past three decades in the US [2]. Among non-melanoma skin cancer patients, it is found in 23% of the patients [3]. Australia has the highest incidence as compared to the rest of the world. This type of cancer is caused primarily by the exposure to the UV rays, coming from the sun [3]. It include different types of histopathological and genetic characteristics. From the aspect of incidence rates, there is a predominance of males over females [3], with the higher incidence observed above the age of 50 years [2]. In the US, incidence of squamous cell carcinoma in men was found to be 81-136 per 100,000 and that of women was found to be 26-59 per 100,000 per year, thus showing a clear predominance in males [4].
Etiology/predisposing factors
SCC develops due to the alteration in the DNA of the squamous cells present in the outer skin layer. Normally, the new cells drive the older cells upwards. towards the surface of the skin, where they die and are discarded off the skin. Due to the DNA alterations, a large number of cells grow and abrupt this process. Various risk factors associated with SCC are as follows:
UV light exposure: Exposure to the UV light is the main cause of squamous cell carcinoma. People who remain exposed to UV light for a considerable period of time are susceptible to develop SCC. UVB rays are usually considered to be more potent than other rays, but usually all the UV rays are harmful. People living in areas that receive bright sunlight throughout the year or those who spend a long duration of time outside, for work or holiday purpose are at higher risk.
Susceptibility to UV light exposure: People with fair skin type have low amount of skin protecting pigment, melanin, which makes them more susceptible to UV light. So, the white people are at higher risk of SCC than African Americans or people belonging to Hispanic race.
Chemical carcinogens: Some chemicals like arsenic and chromium, soot (scrotal cancers in chimney sweeps), tar and pitch oils may cause SCC.
Human papillomavirus infection: Human papilloma virus infection may be a risk factor for SCC.
Ionising radiation: Exposure to ionising radiation may cause SCC.
Immunodeficiency: Immune deficient patient may be more susceptible to SCC.
Chronic inflammation: Individuals having inflammatory diseases like chronic ulcers, chronic sinuses (e.g. osteomyelitis), lupus vulgaris (cutaneous tuberculosis) are more susceptible to SCC.
Genetic conditions: There are some genetic conditions that may contribute to SCC, such as xeroderma pigmentosum and albinism.
Premalignant conditions: Some diseased conditions may be responsible for SCC, like Bowen's disease (skin actinic damage), multiple actinic keratoses (a premalignant lesion on sun-exposed skin), keratoacanthomas (a benign proliferation of squamous epithelium).
Pathophysiology/molecular basis
In this carcinoma, an early stage shows replacement of normal epithelial cells by atypical squamous cells throughout the epidermis, resulting in abnormal growth.
CDKN2A: Mutation and hypermethylation can inactivate CDKN2A, which may be responsible for the development of SCC (Figure 1) [5].
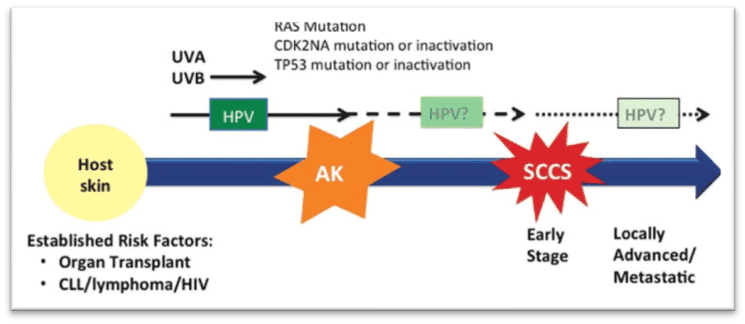
Figure 1. Generation of cutaneous SCC [5]
RAS: Mutations in RAS can cause SCC. About 3-30% of the patients of cutaneous SCC showed mutation in RAS. Some studies have reported theincidence of SCC of the skin, in up to 4-31% of those individuals, who were taking Vemurafenib, Dabrafenib and Sorafenib. BRAF-inhibitors may cause mutation in RAS by paradoxical activation of the MAPK pathway (Figure 1) [5].
TP53: TP53 (transcriptional regulator) is a tumor suppressor gene (TSG), which controls the expression of genes involved in the cell cycle, DNA repair, apoptosis, and senescence. Mutations in TP53 can inactivate it. UVB radiation induced mutation in TP53 gene is demonstrated in 45-60% of cutaneous SCC. This inactivation of p53 is considered to be a critical step in the development of SCC of the skin. Moreover, TP53 mutations may be responsible for the resistance to chemotherapies (Figure 1) [5,6].
p63: p63 gene is critical for the development of stratified epithelial tissues, such as epidermis, and is restricted to the proliferative (basal layer) compartment of the epithelium. The expression of p63 has been identified to be a strong predictor for poorly differentiated SCC of the skin [6].
Ki-67 (MKI67): It is a cell proliferation index marker, that is normally increased in tumors. Ki-67 (MK167) is associated withrecurrence and rapid growth in SCC [6].
CCND1: It is a cell cycle regulator, which is involved in the development of SCC through differentiation and abnormal tissue organization. It is also responsible for the over expression in keratinocyte carcinogenesis [6].
EGFR: EGFR is present in the cell membrane. It is activated by ligand binding process. In many cancers, mutated EGFR may show over expression, as in the SCC of the skin [6].
CDH1 (E-cadherin): CDH1 gene is mainly related to cadherin family of Ca2+ dependent cell-cell adhesion molecules, which induce and maintain intercellular connections. It is involved in carcinogenesis due to the the promoter hypermethylation and decreased expression. Thus, downregulation of CDH1 is associated with tumor progression and metastasis. However, methylation is related to the advanced stage of squamous carcinogenesis in the skin [6].
MMPs: Matrix metalloproteinases (MMPs) are a family of zinc-containing endopeptidases, that are responsible for degradation of many components of the extracellular matrix, and are related to cancer progression, invasiveness and metastasis. In SCC of the skin, immunoexpression of MMP-2 and MMP-9 is correlated with the pathogenesis of SCC [6].
Immunotherapy for squamous cell carcinoma
Monoclonal antibodies (MABs)
Non FDA Approved Monoclonal Antibodies: (Table 1)
Table 1. Non-FDA approved monoclonal antibodies [7-8]
Drugs |
Clinical trial identifier no. |
Phase |
Study Design |
Target |
Cetuximab |
NCT00240682 |
Phase II |
Safety/Efficacy Study Single Group Assignment
Open Label |
EGFR |
Panitumumab |
NCT01129154 |
Phase II |
Efficacy Study, Open Label |
EGFR |
Checkpoint inhibitors
Non FDA approved checkpoint inhibitors: (Table 2)
Table 2. Non-FDA approved checkpoint inhibitors [9]
Drugs |
Clinical trial identifier no. |
Phase |
Study Design |
Target |
Nivolumab |
NCT02327078 |
Phase I, II |
Open Label, Treatment |
PD-1 |
Kinase inhibitors:
Non FDA approved Kinase inhibitors: (Table 3)
Table 3. Non-FDA approved kinase inhibitors [10-14]
Drug |
Clinical trial identifier no. |
Phase |
Study Design |
Target |
Gefitinib |
NCT00126555 |
Phase II |
Efficacy Study/Open Label |
EGFR tyrosine kinase |
Afatinib |
NCT01732640 |
Phase I, II |
Safety/Efficacy Study, Open Label |
EGFR |
Sorafenib |
NCT02035527 |
Phase I, II |
Safety/Efficacy Study, Open Label |
RAF kinase |
Pazopanib |
NCT01716416 |
Phase I |
Safety Study, Open Label |
VEGFR |
Dacomitinib |
NCT01737008 |
Phase I |
Safety/Efficacy Study; Open Label |
EGFR |
Adoptive cell therapy
Non FDA approved Adoptive cell Therapy: (Table 4)
Table 4. Non-FDA approved adoptive cell therapy [15]
Drug |
Clinical trial identifier no. |
Phase |
Study Design |
Target |
|
| |
Natural Killer T-cells |
NCT01801852 |
Phase I |
Safety/Efficacy Study, Single Group Assignment, Open Label |
Squamous cell |
|
mTOR inhibitors
Non-FDA approved mTOR Inhibitors: (Table 5)
Table 5. Non-FDA approved mTOR inhibitors [16]
Drug |
Clinical trial identifier no. |
Phase |
Study Design |
Target |
Everolimus |
NCT01637194 |
Phase I |
Safety Study, Open Label |
FK Binding Protein-12 |
Cytokine therapy
Non-FDA approved Cytokine Therapy: (Table 6)
Table 6. Non-FDA approved cytokine therapy [17]
Drug |
Clinical trial identifier no. |
Phase |
Study Design |
Target |
Interferon alpha-2b |
NCT02218164 |
Phase II |
Safety/ Efficacy Study, Open Label |
Tumor cells |
2021 Copyright OAT. All rights reserv
Conclusion
Squamous cell carcinoma is developed from keratinocytes. Its incidences are more than death cases and most of the incidences are found in Australia. More men are affected by SCC than women. The main cause of cutaneous SCC is exposure to the UV radiation. Immunotherapy has shown to be a promising development in the past few years. The recent activities have increased our understanding of the tumour microenvironment, various immunotherapeutic modalities or combination therapy (like chemotherapy with immunotherapy). Additionally, the effects of such modalities in combination with immunotherapy in cancer patients are still exploratory phase. The complete perspective of the immunotherapy treatment has not been realized yet. Proper preclinical and clinical designs are the important pillars in understanding the future of immunotherapy in treating cancer patients.
References
- Health information (2014) http://www.patient.co.uk/doctor/squamous-cell-carcinoma-of-skin
- Skincare information (2014) http://www.skincancer.org/skin-cancer-information/squamous-cell-carcinoma
- http://www.cancerresearchuk.org/cancer-info/cancerstats/types/skin/incidence/uk-skin-cancer-incidence-statistics#nmsc
- Typesof skin cancer (2014) http://skincancer.dermis.net/content/e04typesof/e151/e152/index_eng.html
- Palyca P, Koshenkov VP, Mehnert JM (2014) Developments in the Treatment of Locally Advanced and Metastatic Squamous Cell Carcinoma of the Skin: A Rising Unmet Need. Asco Educational Book 2014: e397-e404. [Crossref]
- Wusheng Yan, Wistuba II, Emmert-Buck MR, Erickson HS (2011) Squamous cell carcinoma – similarities and differences among anatomical sites. Am J Cancer Res: 275-300. [Crossref]
- Centre Hospitalier of Chartres (2015) Study of Cetuximab in Squamous Cell Carcinoma of the Skin Expressing EGFR (CTXSCC). In: ClinicalTrials.gov, Bethesda (MD): National Library of Medicine (US).
- Cliniquesuniversitaires Saint-Luc- Université Catholique de Louvain; Cliniques Universitaires UCL de Mont-Godinne, Dr Joseph Kerger; Cliniques Saint-Pierre Ottignies, Dr Lionel Duck. Panitumumab (Vectibix®) in Cutaneous Squamous Cell Carcinoma (SCC) (PASCE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- Incyte Corporation (2015) A Study of the Safety, Tolerability, and Efficacy of INCB24360 Administered in Combination with Nivolumab in Select Advanced Cancers. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- National Cancer Institute (2015) Gefitinib in Treating Patients Who Are Undergoing Surgery and/or Radiation Therapy for Locally Advanced or Recurrent Squamous Cell Skin Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- Sidney Kimmel Comprehensive Cancer Center, Vanderbilt-Ingram Cancer Center, National Comprehensive Cancer Network (2015) A Phase I/II Study Afatinib/Carboplatin/Paclitaxel Induction Chemotherapy In HPV-Negative HNSCC. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- Ohio State University Comprehensive Cancer Center, National Comprehensive Cancer Network (2015) Sorafenib Tosylate, Cisplatin, and Docetaxel in Treating Patients With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- Washington University School of Medicine (2015) Pazopanib Plus Cetuximab for Incurable Head and Neck Squamous Cell Carcinoma (HNSCC). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- University Health Network, Toronto (2015) Study of Dacomitinib With Radiotherapy With and Without Cisplatin in Patients With Squamous Cell Carcinoma of the Head and Neck. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- Chinese PLA General Hospital, Han weidong (2015) Autologous Natural Killer T Cells Infusion for the Treatment of Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- Fox Chase Cancer Center, National Cancer Institute (2015) Cetuximab and Everolimus in Treating Patients With Metastatic or Recurrent Colon Cancer or Head and Neck Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- H. Lee Moffitt Cancer Center and Research Institute (2015) Pegylated Interferon Alpha-2b and Capecitabine in Unresectable/Metastatic Cutaneous Squamous Cell. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).

