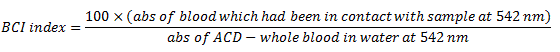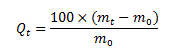Agent with both great blood clotting activity and bone regeneration ability is deserved to replace conventional bone wax. Recently, hydroxyapatite (HA) has attracted interests from researchers with its both hemostatic and bone healing functions. In present work, the blood clotting activity comparisons of HA to other potential bone repairing materials including calcium silicate, calcium combined attapulgite, calcium tripolyphosphate, and chitosan were carried out to show HA as a recommended hemostatic component to replace bone wax. In addition, the impacts of HA synthesis routes on its blood clotting activity were evaluated, indicating increase of surface area as well as active Ca2+ of HA can greatly enhance blood clotting. With these attributes, it is expected HA can be a promising component in fabricating hemostatic materials in orthopedic applications as alternatives to bone wax.
hydroxyapatite, hemostatic agent, bone, blood clotting
Hemostatic agent is critical for successful clinical outcomes in bone defects surgery. Conventionally, beeswax-based bone wax has been used as hemostatic agent. But it is challenged for its poor biodegradation and biocompatibility [1]. Potential alternative hemostatic candidates in orthopedic surgery include both natural polymers such as collagen, cellulose, gelatin etc. and inorganic materials such as zeolite, clays, and silica. However, these materials may have different problems for clinical practice. For example, as shown in a current spinal surgery study on rats, hemostatic polymers may cause undesirable complications such as inflammation and fibrosis [2]. On the other hand, the inorganic materials may be associated with non-biocompatible and/or non-biodegradable nature, as well as hydration related thermal issue [3,4].
In principle, an ideal hemostatic agent for orthopedic applications should not only be able to stop bleeding but also promote bone healing. Recently, hydroxyapatite (HA, Ca10(PO4)6(OH)2) has attracted interests from researchers because of its hemostatic properties, besides its more well-known bone healing function [5,6]. Initially HA was combined with hemostatic polymers to improve their limited osteoconductivity. For example, Hoffmann fabricated a HA/starch/chitosan composite hemostatic material, proposed to be a substitute for bone wax or even as a bone filling material for orthopedic surgery applications [7]. After that, researcher noticed the presence of HA not only improve the composite’s bone regeneration ability, but also enhance its blood clotting activity. Maruyama et al. combined HA with agarose gel and reported the presence of HA can greatly induce activation of blood coagulation and platelets aggregation compared to HA or agarose alone [8]. While Song et al. deposited HA to porous PLGA microspheres and the blood clotting activity was improved in the order of HA content increase [5]. Researchers have suggested blood clotting activity of HA is attributed to its high affinity with plasma proteins such as fibrinogen, and released Ca2+ [8]. Unfortunately, few fundamental studies have been carried out to evaluate the blood clotting activity of HA in comparison to other potential bone repairing materials to highlight its significance as a hemostatic agent in orthopaedic applications. Meanwhile, it is also unclear whether the synthesis routes of HA have impacts on its blood clotting activity. Therefore, in current work we report experimental results of blood clotting activity comparisons of 1) calcium based inorganic bone repairing materials including HA, calcium silicate (CaSiO3), calcium combined attapulgite (Ca-attapulgite, Ca-(Mg,Al)2Si4O10(OH)·4(H2O)), and calcium tripolyphosphate (Ca5(P3O10)2); 2) HA and hemostatic polymers such as chitosan; 3) HA synthesized following different approaches.
Chemicals were purchased from Aladdin China if not specified. HA was hydrothermally synthesized in an autoclave using Ca(OH)2 and Na2HPO4 as reported by our group [9]. Generally, an amount of 0.37 g of Ca(OH)2 was mixed with 300 mL of distilled water to make a suspension. Then 0.71 g Na2HPO4 was added to react with Ca(OH)2. The prepared liquid mixture was magnetically stirred for 15 min. The pH value of the liquid mixture was kept at 10 using 1M NaOH solution. The mixtures were hydrothermally treated in an autoclave for 4hours to obtain HA. CaSiO3 was precipitated via the reaction of tetraethyl orthosilicate (TEOS) and Ca(NO3)2. Briefly, 12 mL NH3.H2O was dissolved in 600 mL distilled H2O with stirring for 30 min. Then, 30 mL TEOS and 31.21g Ca(NO3)2.4H2O were added with vigorous stirring for 3 hours. The products were collected by filtration and washed three times each with distilled H2O and ethanol. Ca-attapulgite was prepared using attapulgite purchased from Zijin Mining, China. The powders were treated by 24 hours acidification using 6M HCl followed by 24 hrs 1M CaCl2 incubation with stirring. Meanwhile Ca5(P3O10)2 was formed by complexation of 1.11 g CaCl2 and 0.123 g Na5P3O10 (STPP) in 100 mL H2O with continued stirring for 30 min. All as prepared powders were characterized using X-ray diffraction (XRD, Rigaku) and transmission electron microscope (TEM, Zeiss).
The blood clotting activity was in vitro measured as blood clotting index (BCI) [10]. Human blood in addition with the anticoagulant citrate dextrose (ACD) (9:1) was used for testing, referred as ACD-whole blood. This blood was kindly provided by Changzhou No.2 People’s Hospital. In brief, 0.09 g of powder was used to contact with 0.27 mL blood sample (0.3mL ACD-whole blood by addition of 0.024 ml CaCl2 (0.2 mol/L)) at 37°C for 10 min. The free blood was collected and diluted into 50 mL for spectrophotometric measurement at 542 nm. The absorbance of 0.25 mL ACD-whole blood in 50 mL deionized water at 542 nm was applied as a reference value. The BCI can be quantified by the following equation:
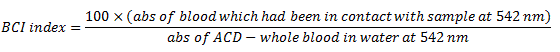
Powders of chitosan, HA and a mixture of both (1:1) were used for BCI testing. Besides, considering HA can be combined with chitosan to fabricate biomimetic bone scaffold [11], comparison between porous chitosan scaffold and HA coated one was also carried out. 600 μL of 0.015 g/mL chitosan solution in well was freeze dried into porous scaffold, which was further incubated into 37°C 1.5x t-simulated body fluid (t-SBF) for 7days with solution replenished every 48 hrs to deposit HA coatings (Table 1). The surface change of chitosan scaffold after SBF incubation was characterized using scanning electron microscope (SEM, Zeiss). The t-SBF is a Tris (C4H11NO6) buffered SBF solution developed by Tas and Bhaduri, closely mimicking the composition of human blood plasma [12]. In present work, the ionic concentrations of t-SBF solution were intensified 1.5times to accelerate HA coating formation. BCI index and the swelling ability of scaffolds in phosphate buffer (PBS) were measured. The swelling ratio of the scaffold at a given time(t), Qt, can be calculated using equation below, where m0 and mt are the weights of the dried and swollen scaffold, and Qt is calculated as grams of water per gram of scaffold.
Table 1. Compositions of 1L 1.5x t-SBF
Order |
Reagent |
Amount |
1 |
NaCl |
9.8184 g |
2 |
NaHCO3 |
3.4023 g |
3 |
KCl |
0.5591 g |
4 |
Na2HPO4 |
0.2129 g |
5 |
MgCl2.6H2O |
0.4574 g |
6 |
1M HCL |
15 mL |
7 |
CaCl2.2H2O |
0.5822 g |
8 |
Na2SO4 |
0.1080 g |
9 |
Tris-Base |
9.0945 g |
10 |
1M HCl |
50 mL |
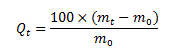
The third part was the study of clotting activity of HA synthesized following different approaches. Sodium hexametaphosphate (Na6P6O18, SHMP), were used to prepare mesoporous HA (HA-HMP) to show the increase of surface area can promote clotting [9]. On the other hand, precipitates (HA-1) from the solution of 11.1 g/L CaCl2 and 1.56 g/L NaH2PO4.2H2O were studied to show whether increase of Ca/P can have significant influence on related blood clotting activity. The XRD and TEM characterizations of these powders were also carried out.
The XRD patterns of as-prepared Ca containing inorganic salts are shown in Figure 1. All powders displayed the characteristics of expected phases. According to the XRD, the synthesized HA and Ca-attapulgite matched the profiles in Jade (PDF # 09-0432 and 20-0958) respectively. While the as-prepared CaSiO3 and Ca5(P3O10)2 were mainly amorphous, like reported before [13,14]. The TEM results of these particles are presented in Figure 2. It was seen that the HA, CaSiO3, Ca—attapulgite and Ca5(P3O10)2 present rod-like, spherical, whisker-like and mesoporous morphology respectively. Among these materials, CaSiO3 was commonly studied as an alternative to HA for bone repairing [15]. Additionally, it also showed ability to absorb proteins like HA [16]. Therefore, it is necessary to compare the hemostatic ability of HA and CaSiO3, thus indicting the reason choosing HA as the potential hemostatic agent instead of CaSiO3. Attapulgite was another silicate material highlighted with its absorption ability and bone repairing potential [17]. The incorporation of Ca2+ to attapulgite was supposed to enhance its clotting activity. Meanwhile, the reason choosing Ca5(P3O10)2 was attributed to the report that polyphosphate can accelerate blood clotting [18] and its self-assembled porous structure [13]. Per the BCI results (Figure 3), among them HA had the best blood clotting activity. This phenomenon could be explained by the facts that HA has a high affinity with plasma proteins such as fibrinogen, and can release Ca2+ to specifically activate prothrombin and coagulation factors to enhance blood clotting [8]. Therefore, HA is recommended as the hemostatic agent for bone defect applications from above 4 pickups.
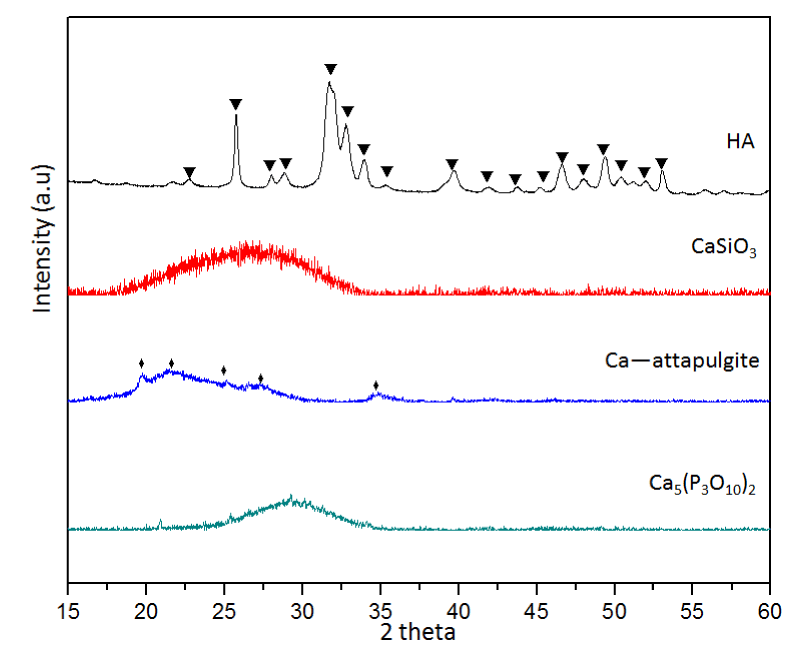
Figure 1. XRD patterns of tested calcium contained inorganic salts, “▼”refers to HA and “♦” refers to attapulgite.
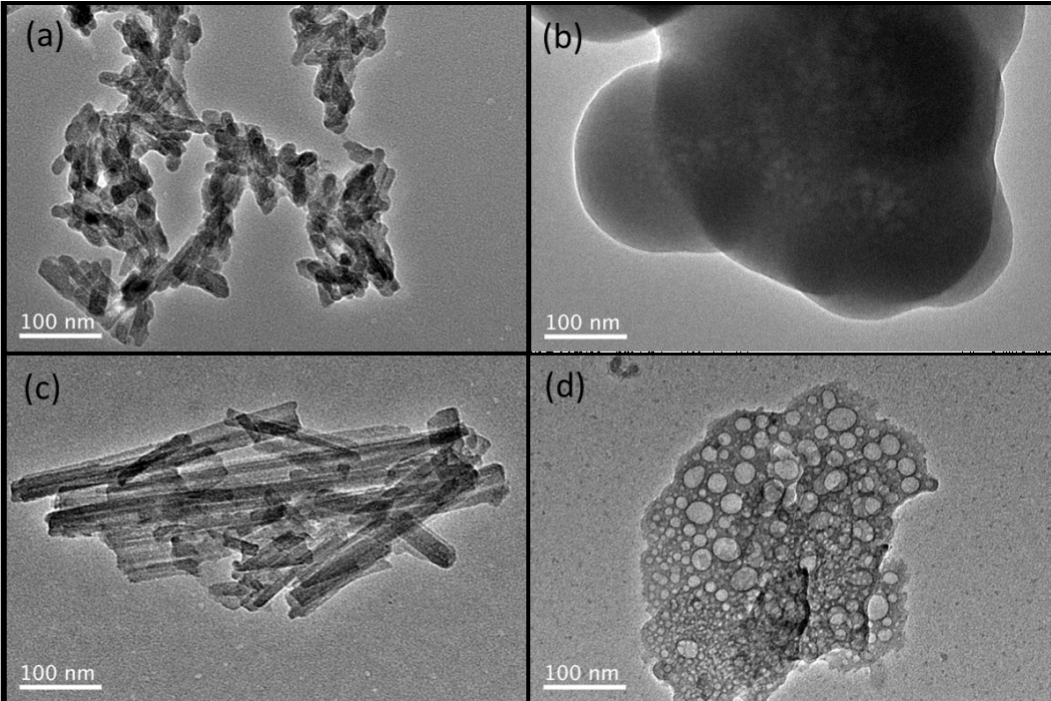
Figure 2. TEM images of (a) HA, (b) CaSiO3, (c) Ca-attapulgite, (d) Ca5(P3O10)2
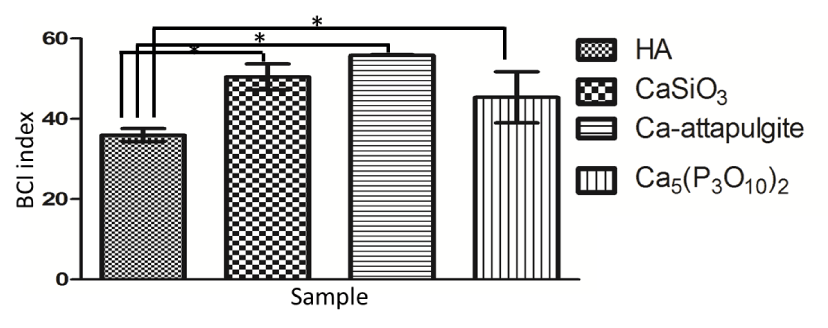
Figure 3. BCI index results of HA, CaSiO3, Ca-attapulgite, and Ca5(P3O10)2 (“*” indicates p < 0.05)
On the other hand, when compared to chitosan powder, HA showed better clotting activity (Figure 4). When chitosan was mixed with HA, its blood clotting activity was comparable to HA instead. This phenomenon could be caused by the combined effects of multiple clotting routes of chitosan and HA. Indeed, chitosan stimulated platelet and erythrocytes aggregation via its amino residue (positively charged surface) [19] and concentrated blood to accelerate clotting via its hydration behavior [20], showing completely different coagulation routes to HA. On the other hand, when HA was coated onto chitosan matrix, the clotting activity was not only depending on the combined effects of chitosan and HA, but also influenced by the amount of blood concentrated by porous scaffold. According to SEM after 7days SBF incubation, HA was successfully deposited to chitosan matrix (Figure 5). Though HA limited the swelling of scaffold (Figure 6a), the BCI index difference between chitosan and HA coated was not significant (Figure 6b). This observation was suggested to be caused by the increase of HA content (49 ± 5 wt.%) and matrix stiffness [21]. As reported by Qiu et al., increasing substrate stiffness led to increased platelet adhesion, spreading, and platelet activation [22].
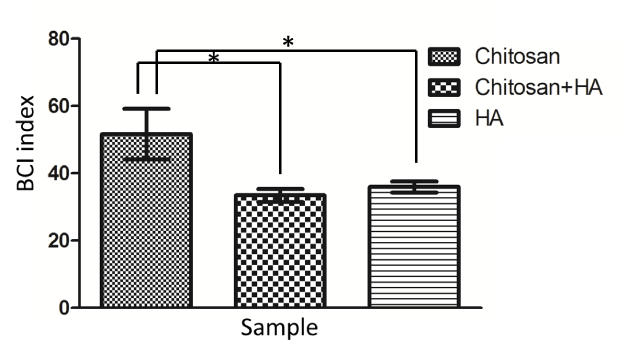
Figure 4. BCI index results of HA, chitosan and a mixture of HA and chitosan
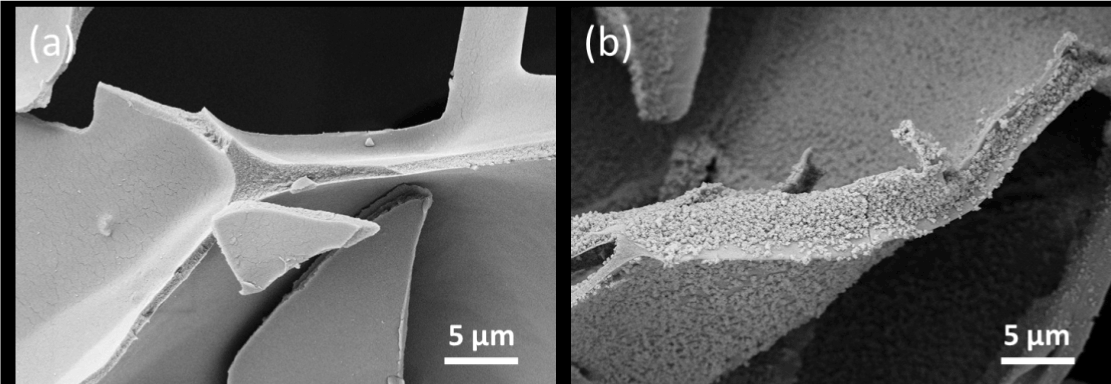
Figure 5. SEM characterization of (a) porous chitosan scaffold; and (b) HA coated chitosan scaffold
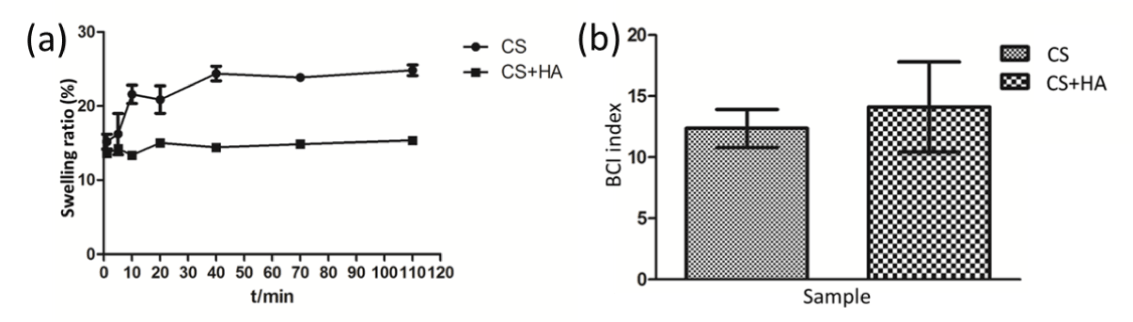
Figure 6. Comparisons between blank and HA coated chitosan scaffolds: (a) swelling ability: and (b) BCI index results
In literature, depending on the phosphate source used as well as hydrothermal condition, the morphology of HA can be tailored [9,23]. It was reported Inorganic condensed phosphates have a high affinity to Ca2+ ions to form complex in aqueous medium. Under hydrothermal condition, condensed phosphates could be hydrolyzed to release orthophosphate subsequently. Therefore, using P6O186- instead of PO43- could result in mesoporous HA, thus changing its clotting activity. The HA-HMP was proved to be HA according to XRD (Figure 7a). And an irregularly shaped and mesoporous morphology was presented (Figure 7b). The increase of surface area enhanced the clotting activity in comparison to regular HA dense particles as expected (Figure 7c).
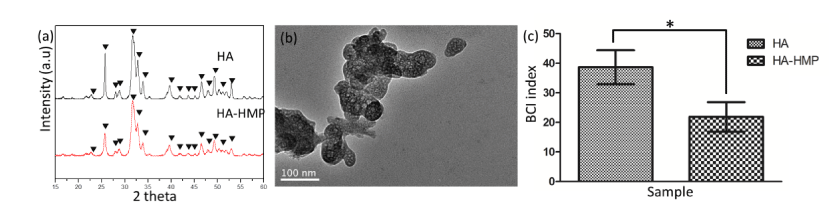
Figure 7. Tests of HA and HA-HMP: (a) XRD of HA and HA-HMP, “▼” refers to HA; (b) TEM of HA-HMP; (c) BCI results of HA and HA-HMP, “*” indicates p < 0.05
On the other hand, the HA-1 with significant increase of Ca/P in reaction solution resulted in formation of phase impurity and a great increase of blood clotting activity. As seen in XRD, HA-1 displayed characteristics of both HA and brushite (CaHPO4.2H2O, PDF#09-0077) (Figure 8a). Consequently, in TEM nanoparticles showed both rod-like and plate-like morphologies (Figure 8b). In the followed BCI test, HA-1 showed much higher blood clotting activity than HA (Figure 8c). It was known fast precipitation of HA caused by strong ionic concentration can induce significant amounts of ions loaded to HA lattice structure [24]. Therefore, in HA-1 a quick release of Ca2+ was expected once in contact with blood to stimulate coagulation cascade. After coagulation, both HA and brushite could induce bone regeneration. This phenomenon provided a possibility to load different ions to HA to help both blood clotting and bone formation. Indeed, different ions such as Mg2+, Zn2+, CO32- have been doped into HA to favor bone formation or even provide anti-bacterial property [25,26]. However, these ions also showed potential to enhance blood clotting in addition to Ca2+. For example, Mg2+ was observed to enhance coagulant activity of factor IXa [27]; Zn2+ was found to be an important cofactor in regulating platelet aggregation and coagulation [28]; while the presence of CO32- in HA could promote blood clotting and protein adsorption [29].

Figure 8. Tests of HA and HA-1: (a) XRD of HA and HA-1, “▼” refers to HA, and “■” refers to brushite; (b) TEM of HA-1; (c) BCI results of HA and HA-1, “*” indicates p < 0.05
In summary, we showed 1) HA is recommended as a potential agent for blood clotting and bone repairing alone or combined with biopolymers; 2) great surface area as well as high amount of active Ca2+ can significantly improve the blood clotting activity of HA. It is wished present work can promote the development of HA based products to replace conventional bone wax.
This work was partially supported by Changzhou Sci & Tech Program (No. CJ20160040); National Natural Science Foundation of China (No. 11532003); The Natural Science Foundation of Jiangsu Province Research of China (No. BK20151181); High-Level Medical Talents Training Project of Changzhou (No. 2016CZLJ004); The Municipal Social Development Project of the Changzhou (No. CJ20160029)
- Nooh N, Abdullah WA, Grawish Mel-A, Ramalingam S, Javed F, et al. (2014) The effects of surgicel and bone wax hemostatic agents on bone healing: An experimental study. Indian J Orthop 48: 319-325. [Crossref]
- Altun I (2016) An Experimental Study of Histopathologic Effects of Hemostatic Agents Used in Spinal Surgery. World Neurosurg 90: 147-153. [Crossref]
- Pourshahrestani S, Zeimaran E, Djordjevic I, Kadri NA, Towler MR (2016) Inorganic hemostats: The state-of-the-art and recent advances. Mater Sci Eng C Mater Biol Appl 58: 1255-1268. [Crossref]
- Nemmar A, Albarwani S, Beegam S, Yuvaraju P, Yasin J, et al. (2014) Amorphous silica nanoparticles impair vascular homeostasis and induce systemic inflammation. Int J Nanomed 9: 2779-89. [Crossref]
- Song L, Sun L, Jiang N, Gan Z (2016) Structural control and hemostatic properties of porous microspheres fabricated by hydroxyapatite-graft-poly (D, L-lactide) nanocomposites. Composites Science and Technology 134: 234-241.
- Hama C, Umeda T, Musha Y, Koda S, Itatani K (2010) Preparation of novel hemostatic material containing spherical porous hydroxyapatite/alginate granules. Journal of the Ceramic Society of Japan 118: 446-450.
- Hoffmann B, Volkmer E, Kokott A, Weber M, Hamisch S, et al. (2007) A new biodegradable bone wax substitute with the potential to be used as a bone filling material. Journal of Materials Chemistry 17: 4028-4033.
- Arimura S, Kawahara K, Biswas KK, Abeyama K, Tabata M, et al. (2007) Hydroxyapatite formed on/in agarose gel induces activation of blood coagulation and platelets aggregation. J Biomed Mater Res B Appl Biomater 81: 456-461. [Crossref]
- Zhou H, Yang M, Zhang M, Hou S, Kong S, et al. (2016) Preparation of Chinese mystery snail shells derived hydroxyapatite with different morphology using condensed phosphate sources. Ceramics International 42: 16671-16676.
- Shih MF, Shau MD, Chang MY, Chiou SK, Chang JK, et al. (2006) Platelet adsorption and hemolytic properties of liquid crystal/composite polymers. Int J Pharm 327: 117-125. [Crossref]
- Zhong C, Chu CC (2012) Biomimetic mineralization of acid polysaccharide-based hydrogels: towards porous 3-dimensional bone-like biocomposites. Journal of Materials Chemistry 22: 6080-6087.
- Jalota S, Bhaduri SB, Tas AC (2006) Effect of carbonate content and buffer type on calcium phosphate formation in SBF solutions. J Mater Sci Mater Med 17: 697-707. [Crossref]
- Zhou H, Hou S, Zhang M, Chai H, Liu Y, et al. (2016) Synthesis of ß-TCP and CPP containing biphasic calcium phosphates by a robust technique. Ceramics International 42: 11032-11038.
- Wu CT, Chang J, Fan W (2012) Bioactive mesoporous calcium-silicate nanoparticles with excellent mineralization ability, osteostimulation, drug-delivery and antibacterial properties for filling apex roots of teeth. Journal of Materials Chemistry 22: 16801-16809.
- Mohammadi H, Hafezi M, Nezafati N, Heasarki S, Nadernezhad A, et al. (2014) Bioinorganics in Bioactive Calcium Silicate Ceramics for Bone Tissue Repair: Bioactivity and Biological Properties. Journal of Ceramic Science and Technology 5: 1-12.
- Maeda H, Kato K, Kasuga T (2016) Adsorption behavior of proteins on calcium silicate hydrate in Tris and phosphate buffer solutions. Materials Letters 167: 112-114.
- Wang Z, Zhao YL, Luo Y, Wang SG, Shen MW, et al. (2015) Attapulgite-doped electrospun poly (lactic-co-glycolic acid) nanofibers enable enhanced osteogenic differentiation of human mesenchymal stem cells. RSC Advances 5: 2383-2391.
- Kudela D, Smith SA, May-Masnou A, Braun GB, Pallaoro A, et al. (2015) Clotting Activity of Polyphosphate-Functionalized Silica Nanoparticles. Angew Chem Int Ed Engl 54: 4018-4022. [Crossref]
- Okamoto Y, Yano R, Miyatake K, Tomohiro I, Shigemasa Y, et al. (2003) Effects of chitin and chitosan on blood coagulation. Carbohydrate Polymers 53: 337-342.
- Wu S, Huang Z, Yue J, Liu D, Wang T, et al. (2015) The efficient hemostatic effect of Antarctic krill chitosan is related to its hydration property. Carbohydr Polym 132: 295-303. [Crossref]
- Caridade SG, Merino EG, Alves NM, Mano JF (2013) Biomineralization in chitosan/Bioglass® composite membranes under different dynamic mechanical conditions. Mater Sci Eng C Mater Biol Appl 33: 4480-4483. [Crossref]
- Qiu YZ, Brown AC, Myers DR, Sakurai Y, Mannino RG, et al. (2014) Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc Natl Acad Sci U S A 111: 14430-14435. [Crossref]
- Qi C, Zhu YJ, Sun TW, Wu J, Chen F (2015) Microwave-Assisted Hydrothermal Rapid Synthesis of Amorphous Calcium Phosphate Mesoporous Microspheres Using Adenosine 5 '-Diphosphate and Application in pH-Responsive Drug Delivery. Chem Asian J 10: 2503-2511. [Crossref]
- Tas AC, Bhaduri SB (2004) Rapid coating of Ti6A14V at room temperature with a calcium phosphate solution similar to 10x simulated body fluid. J. Mater. Res.19: 2742-2749.
- Ahmadzadeh E, Talebnia F, Tabatabaei M, Ahmadzadeh H, Mostaghaci B (2016) Osteoconductive composite graft based on bacterial synthesized hydroxyapatite nanoparticles doped with different ions: From synthesis to in vivo studies. Nanomedicine 12: 1387-1395. [Crossref]
- Swetha M, Sahithi K, Moorthi A, Saranya N, Saravanan S, et al. (2012) Synthesis, Characterization, and Antimicrobial Activity of Nano-Hydroxyapatite-Zinc for Bone Tissue Engineering Applications. J Nanosci Nanotechnol 12: 167-172. [Crossref]
- Morita T, Yoshida M, Yamashita T, Sekiya F (1997) A new cascade theory of blood coagulation: Magnesium (II) is a crucial constituent of the blood coagulation cascade. Thromb. Haemost. P1755.
- Vu TT, Fredenburgh JC, Weitz JI (2013) Zinc: an important cofactor in haemostasis and thrombosis. Thromb Haemost 109: 421-430. [Crossref]
- Takemoto S, Kusudo Y, Tsuru K, Hayakawa S, Osaka A, et al. (2004) Selective protein adsorption and blood compatibility of hydroxy-carbonate apatites. J Biomed Mater Res A 69: 544-551. [Crossref]