Menopausal transition is associated with increasing abdominal obesity and the emergence of many features of the metabolic syndrome. The accumulation of fat in a central distribution (intra abdominal) has emerged as a cardiovascular risk factor independent of overall obesity. The study was carried out to determine the prevalence of abdominal obesity and the components of metabolic syndrome in the north Indian rural and urban; pre and post menopausal women in the age range of 25-60 years. In a cross sectional study comprising of 200 subjects selected from the north Indian rural and urban population equally, 100 of the subjects were premenopausal in the age range of 25-40 years and an equal number of subjects were post menopausal in the age range of 45-60 years. Participants underwent demographic, anthropometric and biochemical measurements. The adult treatment panel 3 (ATP3) criteria was used to classify subjects as having metabolic syndrome. Statistical analysis was performed using SPSS (version 15; SPSS, Chicago, IL). Numerical variables were expressed as means ± SD. 71% of post-menopausal subjects were having higher waist circumference (>88 cm), suggestive of abdominal obesity as compared to 56% of pre-menopausal counterparts. BMI (Body mass index) was in the normal range in both the study subjects. Higher numbers of post-menopausal (41% rural and 43% urban) subjects were having components of metabolic syndrome as compared to (20% rural and 27% urban) pre-menopausal subjects. The total prevalence of metabolic syndrome was also higher in post-menopausal subjects. Abdominal obesity correlates with metabolic risk factors independent of age or menopausal status Menopausal transition by increasing abdominal obesity heightens the risk of metabolic syndrome. Early interventions in the form of life style and dietary changes can lower the risk of metabolic syndrome both in pre and post menopausal subjects.
Menopause, BMI, metabolic syndrome, abdominal obesity, insulin resistance, diabetes mellitus
IHD: Ischemic heart disease, MS: Metabolic syndrome, CVD: Cardio vascular disease, ATP: Adult treatment panel, BMI: Body mass index, WC: Waist circumference, WHR: Waist to hip ratio, TG: Triglycerides, VLDL: Very low density lipoprotein, LDLc: Low density lipoprotein cholesterol, HDLc: High density lipoprotein cholesterol, FBG: Fasting blood glucose
Metabolic syndrome is a cluster of risk factors for type 2 diabetes and cardiovascular disease (CVD), with insulin resistance proposed as a linking factor [1,2]. Metabolic syndrome is increasing in prevalence worldwide, largely attributed to increasing obesity and sedentary lifestyle. Beyond CVD and type 2 diabetes, individuals with metabolic syndrome seemingly are susceptible to other conditions, notably polycystic ovary syndrome, fatty liver, cholesterol gallstones, asthma, sleep disturbances, and some forms of cancer [3].
Cardiovascular disease is one of the leading causes of death among women in the world [4]. It is estimated that half of all cardiovascular events in women are related to the metabolic syndrome [5]. The studies have indicated that women aged more than 55 have a higher incidence of cardiovascular disease than younger women [6-8].
The transition from pre- to post menopause is associated with the emergence of many features of the metabolic syndrome, including- i) increased central (intra abdominal) body fat; ii) a shift toward a more atherogenic lipid profile, with increased low density lipoprotein and triglycerides levels, reduced high density lipoprotein, and small, dense low density lipoprotein particles; iii) and increased glucose and insulin levels [4]. The emergence of these risk factors may be a direct result of ovarian failure or, alternatively, an indirect result of the metabolic consequences of central fat redistribution with estrogen deficiency [4].
ATP III considered the “obesity epidemic” as mainly responsible for the rising prevalence of metabolic syndrome [5].The accumulation of fat in a central distribution (intra abdominal) has emerged as a cardiovascular risk factor independent of overall obesity [9]. Women with high amounts of visceral fat have an excess of cardiovascular mortality and associated metabolic abnormalities [10].
Estrogen promotes the accumulation of gluteo-femoral fat [11], and the loss of estrogen with menopause is associated with an increase in central fat [12]. The higher prevalence of metabolic syndrome in the post menopausal women can be attributed to estrogen induced body fat redistribution.
The results of various previous studies have been conflicting, some studies have shown that there is no difference in cardiovascular risk factors when comparing premenopausal with postmenopausal women [13,14]. Other studies showed that there is a high prevalence of metabolic syndrome among postmenopausal women, which varies from 32.6% to 41.5% [15,16].
Thus, in the light of above mentioned facts, the present study was carried out in the north Indian population including pre and post menopausal women with an aim to determine the-
i) Prevalence of central obesity and the associated metabolic risk factors,
ii) Central role of abdominal obesity in causing cardio- vascular and metabolic complications
iii) Influence of menopause on the emergence of metabolic syndrome,
iv) And the Impact of urbanization on the prevalence of metabolic risk factors
This cross-sectional study was carried out in the North Indian population including a total of 200 healthy women. 100 of them were pre-menopausal in the age range of 25-40 years while the rest of the 100 women were postmenopausal aged between 45-60 years, selected from the rural and urban population equally. The premenopausal women were regularly menstruating, non-pregnant, and non-lactating with no use of hormonal contraception for at least 1 year.
Postmenopausal women who had at least 1-year history of cessation of menses were included. The exclusion criterion was the coexistence of any other serious illness such as secondary hypertension, diabetes mellitus, ischemic heart disease, liver disease, gastro intestinal disorders, renal disease or any other acute or chronic disease; this also included pregnant females, smokers, women taking oral contraceptives, hormone replacement therapy, antidiabetic, antihypertensive or hypolipidemic drugs. Women who were amenorrhoeic due to hysterectomy or cessation of periods other than by a natural cause were identified and excluded from the study.
A questionnaire was completed for each subject. It included demographic information, smoking, menopausal status, consumption of relevant medication especially anti-diabetic agents, anti-hypertensive agents, hypolipidemic drugs and hormone replacement therapy. In addition, food frequency questionnaire and a questionnaire for leisure time behaviour were used for participants. Considering physical activity, the period and frequency of doing each specific activity as leisure in a week were asked. Details of dietary habits were also taken in to account considering the intake of total fat, saturated fat and cholesterol.
The anthropometric examination included measurement of height, weight, waist and hip circumference, and blood pressure (17). BMI was used as a measure of total-body obesity and waist circumference and waist-to-hip ratio (WHR) as measures of central (upper-body or abdominal) obesity [18]. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg.
Weight (kg) to the nearest 0.2 kg was measured with a calibrated (ADD weighing) scale. The height (in meters) of the subjects was determined with a stadiometer to the nearest 0.5 cm. The BMI was calculated as the weight (kg) divided by the height (m) squared (kg/m2). The subjects were classified as per World Health Organization (WHO) criteria based on BMI. The subjects with:
i) BMI of 25-29.9 kg/m2 were considered Grade 1 overweight or simply overweight,
ii) BMI of 30-39.9 kg/m2 as Grade 2 overweight (or obese) and
iii) BMI equal to or greater than 40 kg/m2 were considered as Grade 3 overweight (severe or morbid obese) [19].
Using a flexible metric tape the waist circumference (in centimetres) was assessed at a point midway between the lowest rib and the iliac crest with the subject standing. Waist-to-hip ratio (WHR) was calculated by waist circumference divided by hip circumference.
Systolic and diastolic blood pressure of each parti¬cipant was measured twice using the ausculta¬tory method with a standardized calibrated mercury column-type sphygmomanometer after 10–15 minutes resting in sitting position from the right hand. Two measurements were done for all women at five-minute intervals and the average of the 2 measurements was calculated.
Biochemical methods
All blood specimens were drawn at 8:00 a.m. after a 12-hour fast. Samples were centrifuged within 1 hour of sample collection and the sera frozen immediately at -20°C. Fasting plasma glucose was determined by the glucose oxidase method (Boehringer Mannheim, Mannheim, Germany). Serum lipid and lipoprotein cholesterol levels were measured in fresh serum samples. Serum total cholesterol and triglyceride levels were determined enzymatically (Boehringer Mannheim). Serum HDL cholesterol level was determined enzymatically after precipitation of LDLs and VLDLs with dextran sulphate MgCl2. LDL cholesterol was calculated by the Freidewald formula.
In all the patients besides blood biochemistry, 12 lead E.C.G. was also performed along with complete clinical examination of the patient. A detailed case record was prepared for each patient on a preformed study sheet.
Metabolic syndrome definition
The study subjects were considered to have metabolic syndrome if they had any three or more of the following criteria, according to the NCEP: ATP III criteria [2]:
1) Central obesity: Waist circumference >88 cm
2) Hypertriglyceridemia: Triglycerides ±150 mg/dL or specific medication
3) Low HDL cholesterol: <50 mg/dL or specific medication
4) Hypertension: Blood pressure ±130 mm systolic or ±85 mm diastolic or specific medication
5) Fasting plasma glucose ±110 mg/dL or specific medication or previously diagnosed type 2 diabetes.
The research protocol was approved by the local ethical committee and informed consent was taken from each subject prior to inclusion in the study
Statistical analysis was performed using SPSS (version 15; SPSS, Chicago, IL). Numerical variables were expressed as means ± SD. In bivariate analysis, the Student t test was used. Statistical significance was considered at P < 0.05.
The study subjects were distributed in to two main groups- (I) and (II) of Premenopausal and Postmenopausal women. These study groups (I) and (II) were further subcategorized as A (rural) and B(urban); I (A) and I (B) included rural and urban premenopausal subjects whereas II (A) and II (B) included post menopausal s rural and urban subjects respectively. Each subgroup comprised of 50 subjects.
Base line characteristics of study subjects and influence of menopausal transition
The mean age of the postmenopausal subjects was 57.25 ± 0.80 years as compared to 34.48 ± 0.74 years of pre menopausal subjects. BMI (Body mass index) indicative of general obesity was within the normal range in both the study groups, though the levels were relatively higher in the post menopausal subjects (p<0.01).
Postmenopausal women had significantly larger WC, higher mean WHR, SBP (p<0.001), and DBP (p<0.01) than their premenopausal counterparts (Table 1).
Significantly raised levels of TG, VLDL, and FBG (<0.001) but reduced levels of HDLc (p<0.001) were observed in postmenopausal subjects (Table 1) as compared to premenopausal counterparts.
Influence of urbanization on base line characteristics of study subjects
Table 2 shows the influence of urbanization on base line characteristics of study subjects.
Statistically insignificant variations were observed in the rural and urban groups. The levels of all the parameters in both the study groups (premenopausal as well as post menopausal) were found to be higher in the urban groups as compared to their rural counterparts. The post menopausal subjects were having significantly higher WHR, SP, DP,FBG, Serum TC, TGs, VLDLc, and LDLc, but low HDLc in both rural and urban groups as compared to premenopausal counterparts.
Comparison of metabolic indicators among pre- and postmenopausal women
Table 3 and Figure 1; show the prevalence of components of metabolic syndrome in different study subjects as per NCEP: ATPIII criteria.
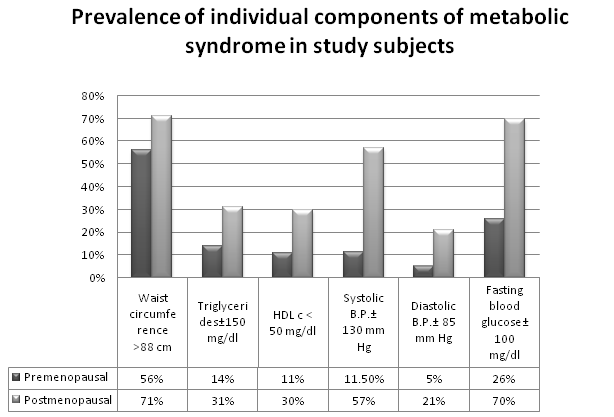
Figure 1. Comparison of prevalence of individual components of metabolic syndrome in premenopausal and postmenopausal subjects
The prevalence of individual components of metabolic syndrome was higher in the post menopausal subjects. Central obesity as measured by waist circumference was also observed in premenopausal subjects. A parallel rise of waist circumference with fasting blood glucose was observed in post menopausal subjects.
The post menopausal subjects in both rural and urban groups were having higher number of components of metabolic syndrome. Waist circumference, systolic blood pressure and fasting blood glucose were found to be higher in post menopausal subjects.
Total prevalence of metabolic syndrome
Figure 2 shows the total prevalence of metabolic syndrome in the study subjects.
The total prevalence of metabolic syndrome was found to be higher in the postmenopausal groups. The urban postmenopausal subjects were having the maximum prevalence (43%).
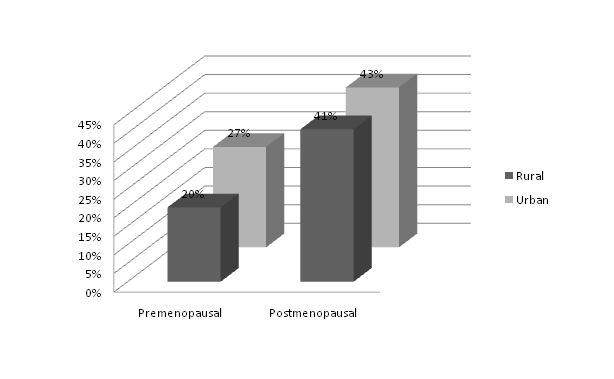
Figure 2. The total prevalence of metabolic syndrome was higher in the post menopausal group. Prevalence was higher in the urban women both in the pre as well post menopausal groups.
Metabolic syndrome, while considered a distinct disorder, includes an increased central distribution of body fat, insulin resistance (IR), dyslipidemia (elevated triglycerides, small dense LDLC particles and reduced HDL-c), elevated blood pressure (BP), and an increased hypercoagulable and proinflammatory state in blood [20]. Obesity and insulin resistance play important pathophysiological role in the etiology of metabolic syndrome [21,22]. The accumulation of fat in a central distribution (intra abdominal) has emerged as a cardiovascular risk factor independent of overall obesity [23]
Table 1. Base line characteristics of study subjects.
Characteristics |
Premenopausal
(Total Rural and Urban) |
Post menopausal
(Total Rural and Urban) |
p value |
Number of subjects |
100 |
100 |
- |
Mean age (years) |
34.48 ± 0.74 |
57.25 ± 0.80 |
<0.0001 |
Body Mass Index (BMI)Kg/m2 |
22.69 ± 2.23 |
24.43 ± 2.34 |
<0.01 |
Waist to Hip ratio (WHR) |
0.79 ± 0.05 |
0.91 ± 0.08 |
<0.001 |
Systolic B.P.(mmHg) |
116.94 ± 6.96 |
141.46 ± 15.62 |
<0.001 |
Diastolic B.P.(mm Hg) |
76.78 ± 5.29 |
80.40 ± 8.67 |
<0.01 |
Fasting blood glucose(mg/dl) |
79 ± 4.56 |
118 ± 6.51 |
<0.001 |
S. Total Cholesterol(mg/dl) |
162.37 ± 25.67 |
224.05 ± 48.89 |
<0.001 |
S. Triglycerides (mg/dl) |
111.85 ± 19.38 |
137.22 ± 40.31 |
<0.001 |
VLDLc (mg/dl) |
22.37 ± 3.88 |
27.44 ± 8.06 |
<0.001 |
LDLc(mg/dl) |
89.47 ± 25.81 |
155.40 ± 49.08 |
<0.001 |
HDLc(mg/dl) |
50.53 ± 6.01 |
41.21 ± 6.87 |
<0.001 |
Continuous data were presented as mean ± standard deviation of mean (SDM). VLDL-C: Very low density lipoprotein-cholesterol, LDL-C: Low density lipoprotein-cholesterol, HDL-C: High density lipoprotein- cholesterol
Table 2 . Base line characteristics of study subjects in different groups (rural and urban).
Characteristics |
Rural |
Urban |
Premenopausal |
Post menopausal |
p value |
Premenopausal |
Post menopausal |
p value |
Number of subjects |
50 |
50 |
- |
50 |
50 |
- |
Body Mass Index (BMI)Kg/m2 |
22.34 ± 1.96 |
24.04 ± 2.36 |
<0.01 |
23.03 ± 2.44 |
24.83 ± 2.27 |
<0.01 |
Waist to Hip ratio (WHR) |
0.776 ± 0.039 |
0.903 ± 0.079 |
<0.001 |
0.809 ± 0.047 |
0.926 ± 0.083 |
<0.001 |
Systolic B.P.(mmHg) |
117.00 ± 6.24 |
139.84 ± 14.62 |
<0.001 |
116.88 ± 6.96 |
141.46 ± 15.62 |
<0.001 |
Diastolic B.P.(mm Hg) |
77.12 ± 5.42 |
80.24 ± 8.79 |
<0.05 |
77.12 ± 5.42 |
80.24 ± 8.79 |
<0.05 |
Fasting blood glucose (mg/dl) |
87 ± 15.63 |
110 ± 26.34 |
<0.001 |
90 ± 14.89 |
119 ± 25.61 |
<0.001 |
S. Total Cholesterol(mg/dl) |
160.54 ± 23.07 |
220.08 ± 44.03 |
<0.001 |
164.20 ± 28.15 |
228.02 ± 53.47 |
<0.001 |
S. Triglycerides (mg/dl) |
111.30 ± 18.93 |
132.10 ± 37.70 |
<0.001 |
112.40 ± 20.00 |
142.34 ± 42.52 |
<0.001 |
VLDLc (mg/dl) |
22.26 ± 3.79 |
26.42 ± 7.54 |
<0.001 |
22.48 ± 4.00 |
28.47 ± 8.50 |
<0.001 |
LDLc(mg/dl) |
88.14 ± 23.32 |
151.46 ± 44.34 |
<0.001 |
90.80 ± 28.26 |
159.33 ± 53.56 |
<0.001 |
HDLc(mg/dl) |
50.14 ± 5.65 |
42.20 ± 6.55 |
<0.001 |
50.92 ± 6.38 |
40.22 ± 7.11 |
<0.001 |
Continuous data were presented as mean ± standard error of mean (SEM). VLDL-C: Very Low density lipoprotein-cholesterol, LDL-C: Low density lipoprotein-cholesterol, HDL-C: High density lipoprotein- cholesterol
Table 3. Prevalence of components of metabolic syndrome in different study subjects (Rural and Urban) as per NCEP: ATPIII criteria.
Characteristic |
Rural (number and percentage of subjects having individual metabolic syndrome component) |
Urban (percentage of subjects having individual metabolic syndrome component) |
Pre menopausal
n (%) |
Post menopausal
n (%) |
Pre menopausal
n (%) |
Post menopausal
n (%) |
Waist circumference >88 cm |
27(54) |
34(68) |
31(58) |
37(74) |
Triglycerides ±150 mg/dL |
5(10) |
12(24%) |
9(18) |
19(38) |
HDL cholesterol: <50 mg/dL |
4(8) |
14(28) |
7(14) |
16(32) |
Systolic Blood pressure ±130 mm Hg |
5(10) |
25(50) |
7(13) |
31(64) |
Diastolic Blood pressure ±85 mm Hg |
2(4) |
10(20) |
3(6) |
11(22) |
Fasting plasma glucose ±100 mg/dL |
12(24) |
34(68) |
14(28) |
36(72) |
A larger number of post-menopausal subjects were having components of metabolic syndrome as compared to pre-menopausal counterparts. Statistically insignificant variations were observed amongst rural and urban subjects with urban subjects having higher values than the rural ones.
Metabolic syndrome and cardio vascular diseases are more common in women above 55 years of age with significant increase in individual risk factors in the postmenopausal phase. Changing hormonal milieu with declining estrogen and alteration of its ratio with testosterone has been implicated as a causal factor for the emergence of metabolic syndrome at menopausal transition [24].
Cross-sectional [25] and longitudinal studies [26] have shown that the menopausal transition is associated with a preferential increase in abdominal adiposity, independent of the effect of age and total body adiposity. During menopause the pattern of hormone secretion changes and gradually causes fat accumulation in visceral tissues of abdomen.
Central obesity (>88 cm of waist circumference) was observed in 68% of the rural and 74% of the urban post menopausal subjects of the present study (Table 3). BMI though higher in the post menopausal subjects (Table 1) was not suggestive of obesity but the waist circumference and waist to hip ratio were more conclusive of prevalence of central obesity amongst post menopausal subjects (Tables 1-3). Similar findings were reported by a number of studies [27,28].
Central obesity was also observed in the premenopausal subjects of present study. 27% of the rural and 31% of the urban premenopausal subjects were found with higher waist circumference (>88 cm - Table 3). Dietary habits, physical inactivity, socioeconomic or genetic background might be the factors to account for central obesity in these subjects.
The subjects with higher waist circumference (both premenopausal and post menopausal) were having higher systolic blood pressure, higher fasting blood glucose and dyslipidemia. A positive correlation was observed between wais circumference and these parameters. These metabolic risk factors were more prevalent in the post menopausal subjects (Table 3). A number of previous studies have also reported higher prevalence of hypertension [29,30], hypercholesterolemia [29], hypertriglyceridemia [30], low HDLc [29] and elevated fasting blood glucose levels [31] amongst postmenopausal subjects. Central obesity progressively increases hepatic and adipose-tissue insulin resistance with the resultant metabolic abnormalities like glucose intolerance, low HDL-C, elevated TG and hypertension [32,33].
2021 Copyright OAT. All rights reserv
The total prevalence of metabolic syndrome was higher in postmenopausal subjects. In the rural and urban post menopausal groups it was 41% and 43% respectively in comparison to 20% and 27% of the premenopausal subjects (Figure 2). These results were consistent with many of previous studies [34-38], where post-menopausal women were found to be at higher risk of MS than pre-menopausal women.
Rural and urban variations in the components of metabolic syndrome in both pre and post menopausal subjects of our study were in accordance with the reports of other studies [39,40]. These differences in prevalence of metabolic syndrome might be due to socioeconomic and environmental differences, dietary factors and lifestyle. Physical activity appears protective for obesity, high blood pressure, and low HDL-c [41]. Physical activity is higher in the rural population. The sustained excess of energy-dense foods, an increasingly sedentary lifestyle—attributed in part to urbanization, which limits the opportunities for physical activity, might be the major causes of energy imbalance leading to obesity in the urban women.
Abdominal obesity per se is the leading factor for metabolic syndrome. Although menopausal transition, by changing the hormonal milieu, is associated with increasing tendency for visceral fat deposition and the associated complications, but rising waist circumference, indicative of abdominal obesity, at any age, irrespective of menopausal status should be considered an alarm for the onset of metabolic syndrome. Early interventions promoting physical activity, life style, and dietary modifications to prevent abdominal obesity should be undertaken to reduce the risk of metabolic syndrome. Waist measurement can be an effective screening tool to detect the prevalence of metabolic syndrome.
None
Namrata Chhabra- Drafting of manuscript, analysis and interpretation of the data, overall responsibility, Kuldip Sodhi- Concept and design of the research and final approval of the script, Sahiba Kukreja- Collection of data, funding and critical revision of manuscript, Sahil Chhabra- Drafting of manuscript, analysis and interpretation of the data, Sarah Chhabra- Drafting the manuscript and statistical analysis, Kavish Ramessur- Statistical Analysis and interpretation of data. All authors meet the criteria for authorship stated in the Uniform requirement for manuscripts submitted to biomedical journals.
- 1. Alberti KG, Zimmet P, Shaw J (2006) Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469-480. [Crossref]
- 2. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001). Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) C. JAMA 285: 2486–2497. [Crossref]
- 3. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C ( 2004) Definition of Metabolic Syndrome- Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation 109: 433-438. [Crossref]
- 4. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, et al. (2009) Heart disease and stroke statistics—2009 update. A report from the American heart association statistics committee and stroke statistics subcommittee. Circulation 119: 480–486.
- 5. Carr MC (2003) The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404-2411. [Crossref]
- 6. Lerner DJ, Kannel WB (1986) Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J 111: 383-390. [Crossref]
- 7. Ford ES, Giles WH, Dietz WH (2002) Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 287: 356–359. [Crossref]
- 8. Rosamond W, Flegal K, Friday G, Furie K, Go A, et al. (2007) Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115: e69-171. [Crossref]
- 9. Kannel WB, Cupples LA, Ramaswami R, Stokes J 3rd, Kreger BE, et al. (1991) Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol 44: 183-190. [Crossref]
- 10. Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, et al. (1984) Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 289: 1257-1261. [Crossref]
- 11. Krotkiewski M, Björntorp P, Sjöström L, Smith U (1983) Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 72: 1150-1162. [Crossref]
- 12. Poehlman ET, Toth MJ, Gardner AW (1995) Changes in energy balance and body composition at menopause: a controlled longitudinal study. Ann Intern Med 123: 673-675. [Crossref]
- 13. Peters HW, Westendorp IC, Hak AE, Grobbee DE, Stehouwer CD, et al. (1999) Menopausal status and risk factors for cardiovascular disease. J Intern Med 246: 521-528. [Crossref]
- 14. Kannel WB, Hjortland MC, McNamara PM, Gordon T (1976) Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 85: 447-452. [Crossref]
- 15. Chedraui P, Hidalgo L, Chavez D, Morocho N, Alvarado M, et al. (2007) Quality of life among postmenopausal Ecuadorian women participating in a metabolic syndrome screening program. Maturitas 56: 45-53. [Crossref]
- 16. Ding QF, Hayashi T, Zhang XJ, Funami J, Ge L, et al. (2007) Risks of CHD identified by different criteria of metabolic syndrome and related changes of adipocytokines in elderly postmenopausal women. J Diabetes Complications 21: 315-319. [Crossref]
- 17. World Health Organization (1999) Diabetes and Non-Communicable Disease Risk Factor Surveys: A Field Guide. Geneva, World Health Org (WHO/NCD/NCS/99-1).
- 18. [No authors listed] (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894: i-xii, 1-253. [Crossref]
- 19. World Health Organization (1998). Prevention and Management of the Global Epidemic of Obesity. Report of the WHO Consultation on Obesity. Geneva, Switzerland: WHO (Technical Report Series, No. 894).
- 20. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285: 2486-2497. [Crossref]
- 21. Ding QF, Hayashi T, Zhang XJ, Funami J, Ge L, et al. (2007) Risks of CHD identified by different criteria of metabolic syndrome and related changes of adipocytokines in elderly postmenopausal women. J Diabetes Complications 21: 315-319. [Crossref]
- 22. Lobo RA (2008) Metabolic syndrome after menopause and the role of hormones. Maturitas 60: 10-18. [Crossref]
- 23. Kannel WB, Cupples LA, Ramaswami R, Stokes J 3rd, Kreger BE, et al. (1991) Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol 44: 183-190. [Crossref]
- 24. Pandey S, Srinivas M, Agashe S, Joshi J, Galvankar P, et al. (2010) Menopause and metabolic syndrome: A study of 498 urban women from western India. J Midlife Health 1: 63-69. [Crossref]
- 25. Gavaler JS, Rosenblum E (2003) Predictors of postmenopausal body mass index and waist hip ratio in the oklahoma postmenopausal health disparities study. J Am Coll Nutr 22: 269-276. [Crossref]
- 26. Usoro CAO, Onyeukwu CU, Nsonwu AC (2007) Biochemical bone turnover markers in postmenopausal women in Calabar municipality. Asian J. Biochem 2: 130-135.
- 27. Zamboni M, Armellini F, Milani MP, De Marchi M, Todesco T, et al. (1992) Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables and their inter-relationships. Int J Obes Relat Metab Disord 16: 495-504. [Crossref]
- 28. Björkelund C, Lissner L, Andersson S, Lapidus L, Bengtsson C (1996) Reproductive history in relation to relative weight and fat distribution. Int J Obes Relat Metab Disord 20: 213-219. [Crossref]
- 29. Expert Panel on Detection Evaluation THBCA: Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J-AM MED ASSOC 285: 2486-2497.
- 30. Figueiredo Neto JA, Figuerêdo ED, Barbosa JB, Barbosa Fde F, Costa GR, et al. (2010) Metabolic syndrome and menopause: cross-sectional study in gynecology clinic. Arq Bras Cardiol 95: 339-345. [Crossref]
- 31. Ford ES, Li C, Sattar N (2008) Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 31: 1898-1904. [Crossref]
- 32. Pascot A, Després JP, Lemieux I, Bergeron J, Nadeau A, et al. (2000) Contribution of visceral obesity to the deterioration of the metabolic risk profile in men with impaired glucose tolerance. Diabetologia 43: 1126-1135. [Crossref]
- 33. Hamdy O, Porramatikul S, Al-Ozairi E (2006) Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2: 367-373. [Crossref]
- 34. Lin WY, Yang WS, Lee LT, Chen CY, Liu CS, et al. (2006) Insulin resistance, obesity, and metabolic syndrome among non-diabetic pre- and post-menopausal women in North Taiwan. Int J Obes (Lond) 30: 912-917. [Crossref]
- 35. Eshtiaghi R, Esteghamati A, Nakhjavani M (2010) Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 65: 262-266. [Crossref]
- 36. Pandey S, Srinivas M, Agashe S, Joshi J, Galvankar P, et al. (2010) Menopause and metabolic syndrome: A study of 498 urban women from western India. J Midlife Health 1: 63-69. [Crossref]
- 37. Ebrahimpour P, Fakhrzadeh H, Heshmat R, Ghodsi M, Bandarian F, et al. (2010) Metabolic syndrome and menopause: A population-based study. Diab Metab Syndr 4: 5–9. ..
- 38. Heidari R, Sadeghi M, Talaei M, Rabiei K, Mohammadifard N, et al. (2010) Metabolic syndrome in menopausal transition: Isfahan Healthy Heart Program, a population based study. Diabetol Metab Syndr 2: 59. [Crossref]
- 39. Larsson B, Bengtsson C, Bjorntorp P, Lapidus L, Sjöstrom L et al. (1992) Is abdominal body fat distribution a major explanation for the sex difference in the incidence of myocardial infarction? The study of men born in 1913 and the study of women, Goteborg, Sweden. Am J Epidemiol 135: 266–273.
- 40. Ntandou G, Delisle H, Agueh V, Fayomi B (2009) Abdominal obesity explains the positive rural-urban gradient in the prevalence of the metabolic syndrome in Benin, West Africa. Nutr Res 29: 180-189. [Crossref]
- 41. Babu K P S, Murthy A G S, B G, Hamsaveena (2013) Metabolic Syndrome among Urban and Rural Women Population - A Cross Sectional Study. J Clin Diagn Res 7: 1938-1940. [Crossref]
Editorial Information
Editor-in-Chief
Masayoshi Yamaguchi
Emory University School of Medicine
Article Type
Research Article
Publication history
Received: August 15, 2014
Accepted: September 07, 2014
Published: September 20, 2014
Acknowledgements
We are thankful to Dr. N.S.Munshi (Professor and Head Department of Obstetrics and Gynecology) and Dr. N.N. Munshi (Professor and Head Department of Pediatrics), SSR medical college, Mauritius, for their unconditional support and guidance.
Copyright
©2014 Chhabra N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Chhabra N, Sodhi K, Kukerja S, Chhabra S, Chhabra S, et al. (2014). High waist circumference-A potential risk factor for premature metabolic syndrome in women irrespective of menopausal status. Integr Mol Med, 1: DOI: 10.15761/IMM.1000104


