This study is about Forearm Autogenous Arteriovenous Fistula creation using the operative microscope magnification in adult patients. Early thrombotic occlusion occurred within 24 hours in one patient (0.86%) for distal radial-cephalic AVFs and 0% for forearm proximal AVFs). No attempt at revision was made and this patient received a new forearm RCAVF. Non-maturation occurred in 6 patients (6.9%) and in 2 patients (9.5%) respectively. Interventional treatment by means of percutaneous transluminal angioplasty (PTA) resulted in maturation in 100% of patients. No post-operative complication occurred.
arteriovenous fistula, microscope, haemodialysis
Arteriovenous fistulas (AVFs) are considered the gold standard for haemodialysis vascular access. A mature fistula lasts for a prolonged period of time, has a superior patency, low complication rates, low incidence of revisions, and improves survival on dialysis [1]. The distal and proximal radio-cephalic fistula (RCAVF) is still the best site for an internal AVF. Unfortunately, RCAVFs have been associated with higher incidence of early thrombosis and failure to mature (early or primary failure) (20-50%) [2,3].
A recent systematic review placed patency rates at 60% after 1 year if primary failures were included [4]. With proper patient selection some authors have reported a reduction in the incidence (7%) of early thrombosis; however, without early interventions the non-maturation rates in the RCAVF continues to remain high (33%) [5,6]. To minimize the failure rate, is been proposed the use of an operative microscope to create or salvage an AVF for haemodialysis [7,8]; in fact, microsurgery and preventive haemostasis have been reported to give excellent results in a paediatric [9,10] and adult population with an early failure rate of 5-10% and 11% respectively [11,12]. We report ours results about early failure in distal and proximal forearm autogenous AV fistulae performed with microsurgical instruments and surgical microscope.
This study includes 136 adult patients. We created at our institution over a 2.5-year period (June 2008- December 2010) in new patients 115 radio-cephalic wrist arteriovenous fistulae (RCAVF) and 21 higher or mid-forearm radio-cephalic fistulae (FRCAVF) (Table 1 and 2).
Table 1. Main demographic and clinical features.
Number of patients |
136 |
Mean age (y) |
64 ± 23 |
M/F |
72/64 |
Age > 65 years |
74 (64.3%) |
Diabetes mellitus |
43 (31.6%) |
Hypertension |
67 (49.5%) |
Obesity (BMI > 30) |
38 (28.6%) |
Table 2. Preoperative radial artery diameter for distal RCAVF
>1.6 mm |
91 (79.1%) |
< 1.6 mm |
24 (20.9%) |
Preoperative examination
All patients were pre-operatively examined by clinical examination performed by the same surgeon who later carried out the operation. The ultrasound examination was made by a skillful operator in ultrasound technique only in cases in which objective examination gave no steady information and always in diabetic, obese, vasculopathic patients or with previous AVF interventions.
The criterion for a distal access creation was the presence of arterial and venous vessels with no sign of stenosis; radial artery diameter less than 1.6 mm wasn’t limiting factor. In case of ultrasound evidence of inadequate distal vessels we chose to perform a FRCAVF. When pre-operative assessment by clinical examination and duplex ultrasound make it impossible to create a forearm fistula, it is our common practice to perform an elbow AVF.
Operative procedure
All procedures were carried out from the same 30 years experienced interventional nephrologist. The majority of access was performed under local anesthesia. Local-regional anesthesia was chosen in only 3 patients and general anesthesia in only a anxious patient.
The exposure of the artery and vein was performed under loupe magnification (3.5x); the anastomosis was performed using operative microscope (magnification up to x 12) mod. Zeiss.
Prophylactic antibiotics were not used, in accord with Lewis experience [13].
Loupe phase
For the creation of a distal RCAVF, a 3-cm longitudinal incision through the skin was made halfway between the cephalic vein and the radial artery at the wrist. The cephalic vein was dissected first. The dissection was conducted up to the bifurcation of the vein at the dorsal aspect of hand. At this site, the cephalic vein is joined by a tributary (the radial accessory vein) that often drains medial aspect of the back of the hand. This dorsal tributary was normally legated and sectioned for an adequate mobilization.
It is of the utmost importance to prevent torsion and kinking during handling of the vein because the vein is located above the deep fascia while the radial artery is located deep to this fascia. The transposition of the artery over the deep fascia in side-to-side anastomosis or the suture of the posterior wall of the vein and the anterior wall of the artery in end-to-side anastomosis can prevent torsion and the incidence of juxta anastomotic stenosis.
The vessel is only handled by the adventitia. A thin catheter or a blunt needle were introduced into the distal segment of the vein, or into the radial accessory vein and 10 to 20 ml of a warmed (37°C) heparin saline solution was gently injected against proximal digital pressure resistance to dilate the vein.
A 3-cm segment of the radial artery was exposed so that proximal and distal vascular bands could be applied, transecting and legating all its small branches.
Operative microscope phase
At this point is utilized the operating microscope. The artery and vein were approximated and positioned to ensure no kinking at the entrance to the subcutaneous tissue, and with previous atraumatic microvascular clips application, a 10-mm arteriotomy and venotomy were performed with an ophthalmic knife N.20 (Figure 1)
Figure 1. Venotomy with knife n° 20
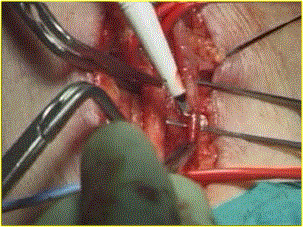
Particular care was taken not to damage the posterior wall of the artery and the vein. The incision was completed with microsurgical scissors. In case of a radial artery internal diameter of less than 1,6 mm, a thin catheter or blunt needle was introduced proximally and distally for injection of a warmed (37°C) heparin saline solution.
In case of side-to-side anastomosis, two cardinal stitches were made in the proximal and distal corner of the anastomosis and a side-to-end arterial-venous suture was made using from 2 to 4 separate 7/0 or 8/0 gore-tex running sutures, depending on the calibre of the vein. Another suture technique we performed frequently is that proposed by Tellis et al. [14] (Figure 2).
Figure 2. One running suture anastomosis
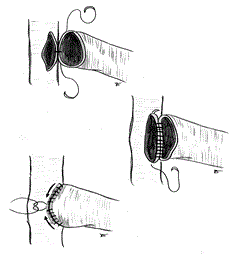
The thecnique of Tellis is a very simple to construct side-to-side and side-to-end anastomosis. Instead of starting the suture in both corners, the suture starts in the middle of the arterial and venous back wall and is then continued to the proximal angle, and down to the middle of the anterior wall. The distal suture is performed afterwards. This “open” technique gives excellent vision of the arterial and venous lumen and is extremely advantageous in handling very small vessels (Figure 3).
Figure 3. Radio-cephalic side-to-side anastomosis (operative microscope view)
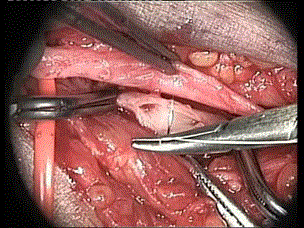
We often used a branch patch technique for end-to-site radio cephalic fistula construction. At the junction with the dorsal tributary, the cephalic vein has a larger calibre and usually runs closer toward the upper part of the distal radial artery. This option is proper when the cephalic vein at the wrist is of small calibre (Figure 4).
Figure 4. Radio-cephalic end-to-site anastomosis
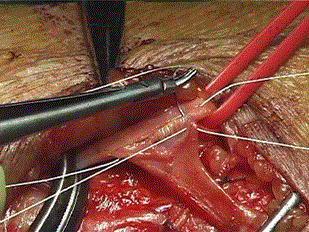
After the release of the vascular bands, pulsation and thrill were immediately palpated at the site of the fistula and the murmur is appreciated with a sterile phonoscope. It is very important to ascertain that the vein is not stenosed at the upper limit of the dissection.
Before proceeding, is mandatory to ascertain that AVF is well functioning. Often some minutes are needed to appreciate a good thrill and murmur. This delay occurs generally because of arterial or venous spasm. In case of persistent spasm we rinse the vessels from outside with warmed saline, and infuse quickly on the peripheral vein previously isolated a solution with 500 mg to 1000 mg of acetylsalicylic acid. If this alone is not effective, we use a Fogarty catheter with extreme caution. In case of Ht values superior to 35% (in the 50% of cases in our experience) acetylsalicylic acid is systematically infused at the dose of 500 mg e.v.
The operating microscope was removed from the field. In the side-to-side anastomosis the distal end of the vein is legated to avoid venous hypertension, creating a functional side-to-end-anastomosis. The subcutaneous tissues not closed with suture and the skin was approximated with a running subcuticular suture of monocryl. Sutures were removed on the 14th post-operative day.
Early thrombotic occlusion occurred within 24 hours in one patient (0.86%) for distal radial-cephalic AVFs and 0% for forearm proximal AVFs). No attempt at revision was made and this patient received a new forearm RCAVF. Non-maturation occurred in 6 patients (6.9%) and in 2 patients (9.5%) respectively. Interventional treatment by means of percutaneous transluminal angioplasty (PTA) resulted in maturation in 100% of patients.
No post-operative complication occurred.
Standard magnification in microsurgery is accomplished with the operating microscope, and Loupes are considerate by the microsurgical community as technically less safe. Instead, in “macro-microsurgery”, for vascular access, loupes are considerate safe; only in very young children use of the operating microscope and microsurgical techniques for the creation of AVF is recommended [9].
Guidelines suggest attempting to create an AVF at the most appropriate distal arm site. In practice, this means choosing the more proximal radio cephalic site before considering a brachiocephalic AVF [15].
However, high rates of RCAVFs thrombose directly after operation or not adequately function due to failure of maturation in adult HD patients.
Primary failure (PF), defined as a complication of the AVF before the first successful cannulation for hemodialysis treatment, is mostly caused by thrombosis in the anastomotic area and by anastomotic and juxta-anastomotic vein stenosis. Juxta-anastomotic stenosis in forearm fistulas appear to arise in the surgical hinge region where the vein is mobilized; this segment of vein is subject to surgical trauma with possible desiccation, crush injury, kinking, spasm, or other insults during fistula creation.
Most episodes of PF relate to errors committed during the planning and execution of the operative procedure. Precise and non-traumatic handling and suturing of vessels is the key point for a correct anastomosis and the critical step towards improving maturation of AVF; even minimal errors are not tolerated [6].
In our series, we report the outcomes of creation of RCAVF using the operative microscope by a single surgeon in adult HD patients dialyzed at a single center. Ours results are similar to Shemesh et al. [16] with a early failure rate (6.8%) in seventy-three distal AVF (42.6% patients diabetic).
The extreme precision allowed by the use of surgical microscope offers many advantages such as precise handling of vessels, suture positioning and sharp intima-to-intima vessel–wall apposition and favors the respect of vessel anatomy, and thereby the functional role played by the endothelial cell layer in fistula maturation [12].
2021 Copyright OAT. All rights reserv
We did not adopt the upper-arm ischemia technique with inflatable tourniquet [7,12]. Despite the described advantages of this technique, mainly the avoidance of arterial spasm, minimal haemostasis, safer dissection of the main vessels, and the absence of clamping, we are not accustomed to using such devices. Therefore, we preferred to dissect the vessels with minimal trauma and legate all the small collateral branches.
Operative microscope assistance allows creating a vast majority of distal radio cephalic fistulae with very low incidence of failures and complications, including diabetics, women, older patients, patients obese and with peripheral vascular disease. The same results we obtained in proximal forearm AVFs. The need for grafts and central venous catheters as first vascular access is reduced even in obese patients.
This technique allows a greater utilization of distal vessels, a sparing of vascular patrimony, a lower cardiac load and a lower central vascular catheter insertion rate. Unfortunately, because of an intellectual attitude this technique is refused (also, often, for the unavailability of the surgical microscope) by the vast majority of operators.
For best results possible to allow a well-functioning vascular access and durable, with consequent advantages, is not only necessary to perform a thorough preoperative mapping and careful monitoring, but use a surgical technique the more accurately as possible using the higher magnification of the operatory field (10-12.5 x). Our findings support use of the surgical microscope and microsurgical instruments for the creation of a forearm AVF in all patients, as asserted by Bourquelot and Pirozzi [17].
- Beathard GA (2010) Creating an arteriovenous fistula for hemodialysis.
- Palder SB, Kirkman RL, Whittemore AD, Hakim RM, Lazarus JM, et al. (1985) Vascular access for hemodialysis. Patency rates and results of revision. Ann Surg 202: 235-239. [Crossref]
- Kherlakian GM, Roedersheimer LR, Arbaugh JJ, Newmark KJ, King LR (1986) Comparison of autogenous fistula versus expanded polytetrafluoroethylene graft fistula for angioaccess in hemodialysis. Am J Surg 152: 238-243. [Crossref]
- Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, et al. (2014) Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 63: 464-478. [Crossref]
- Tordoir HM, Rooyens P, Dammers R, Van der Sande FM, De Haan M, et al. (2003) Prospective evaluation of failure modes in autogenous radiocephalic wrist access for haemodialysis. Nephrol Dial Transplant 18: 378-383. [Crossref]
- Konner K (2002) The anastomosis of the arteriovenous fistula-common errors and their avoidance. Nephrol Dial Transplant 17: 376-379. [Crossref]
- Bourquelot P, Raynaud F, Pirozzi N (2003) Microsurgery in children for creation of arteriovenous fistulas in renal and non-renal diseases. Ther Apher Dial 7: 498-503. [Crossref]
- Bourquelot P (2006) Vascular access in children: the importance of microsurgery for creation of autologous arteriovenous fistulae. Eur J Vasc Endovasc Surg 32: 696-700. [Crossref]
- Bagolan P, Spagnoli A, Ciprandi G, Picca S, Leozappa G, et al. (1998) A ten-year experience of Brescia-Cimino arteriovenous fistula in children: technical evolution and refinements. J Vasc Surg 27: 640-644. [Crossref]
- Chand DH, Bednarz D, Eagleton M, Krajewski L (2009) A Vascular Access Team Can Increase AV Fistula Creation in Pediatric ESRD Patients: A Single Center Experience. Semin Dial 22: 679-683. [Crossref]
- Konner K (1999) A primer on the av fistula-Achilles’ heel, but also Cinderella of haemodialysis. Nephrol Dial Transplant 14: 2094-2098. [Crossref]
- Pirozzi N, Apponi F, Napoletano AM, Luciani R, Pirozzi V, et al. (2010) Microsurgery and preventive haemostasis for autogenous radial-cephalic direct wrist access in adult patients with radial artery internal diameter below 1.6 mm. Nephrol Dial Transplant 25: 520-525. [Crossref]
- Lewis CG, Wells MK, Jennings WC (2003) Avoidance of prophylactic antibiotics in creation of native arteriovenous fistulas. Nephrol Dial Transplant 32: 306-308.
- Tellis VA, Veith FJ, Sobermann RJ, Freed SZ, Gliedman ML (1971): Internal arteriovenous fistula for hemodialysis. Surg Gynecol Obstet 132: 866-870. [Crossref]
- Vascular Access Work Group (2006) Clinical practice guidelines for vascular access. Am J Kidney Dis 1: S248–S270. [Crossref]
- Shemesh D, Zigelman C, Olsha O, Alberton J, Shapira J, et al. (2003) Primary forearm arteriovenous fistula for hemodialysis access- an integrated approach to improve outcomes. Cardiovasc Surg 11: 33-41. [Crossref]
- Bourquelot P, Pirozzi N (2014) Tips and tricks in creation of forearm arteriovenous fistulae. J Vasc Access 7: S45-S49. [Crossref]




