Background
Tolvaptan (TLV) reportedly improves quality of life and averts worsening renal function in patients with congestive heart failure (HF). However, current unrestricted and prolonged TLV use is controversial because its efficacy outcome has not been well documented. We discuss the characteristics associated with outpatient TLV use in patients with heart failure.
Methods
We retrospectively studied 41 patients with acute decompensated heart failure (ADHF) that was treated with TLV. We identified 6 patients requiring outpatient TLV continuation (CONT group) and 35 patients for whom TLV was interrupted during hospitalization (INT group).
Results
Significantly more patients in the CONT group had at least history of ≥ 2 rehospitalization, dilated cardiomyopathy, device implantation, and combined thiazide diuretic treatment than those in the INT group. The Kaplan-Meier estimate of the event rate for all-cause death or HF rehospitalization at 1 year was significantly higher in patients in whom TLV was considered ineffective (log rank p=0.046). In univariate analysis, concomitant device therapy (OR=2.65; 95% CI, 0.85 – 82.9), thiazide use (OR=4.11; 95% CI, 0.67-24.97; p < 0.01), and prior multiple (≥ 2) hospital readmissions for HF (OR=2.73; 95% CI, 0.87 - 8.52) were significantly associated with outpatient TLV use. Multiple logistic regression model showed that only multiple hospital readmission was a covariate to outpatient TLV use (OR=24.55; 95% CI, 1.96 - 306.19).
Conclusion
These findings indicate that outpatient TLV use may be necessary for patients with refractory HF who experience multiple exacerbations.
aquaretic vasopressin antagonist, decongestion, diuretic resistance
Decongestion is a vital part of initial management for acute decompensated heart failure (ADHF) [1]. Prompt fluid removal is the most effective approach to ameliorate patient symptoms such as cardiac dyspnea. Moreover, decongestion provides more than a palliative benefit because the lack of adequate symptom relief with effective decongestion has been associated with longer hospital stays and increased mortality in ADHF [2]. Diuretics have been conventionally utilized in daily clinical practice for pharmacological fluid excretion in ADHF. However, diuretics have multiple challenges in heart failure (HF) management [3]. Potential risks of using loop diuretics include stimulation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system, electrolyte disorders, and low cardiac output because of excessive negative intravascular water balance [3]. Consequently, impaired response to diuretic therapy, termed diuretic resistance, is commonly encountered in ADHF patient management [4].
Tolvaptan (TLV), an aquaretic vasopressin V2 antagonist, has been reported to improve quality of life [5] and short-term clinical outcome [6], and to avert worsening renal function (WRF) in hospitalized patients with HF [7]. Since its approval for treatment of HF in 2010 [8], this drug has been increasingly administered not only for refractory HF with inadequate response to traditional diuretics but also for the management of relatively mild HF cases in Japan. However, unrestricted and prolonged TLV use on an outpatient basis is controversial because its efficacy in this setting is still unclear [9]. In this study, we discuss the factors necessitating outpatient TLV continuation in hospitalized patients with HF.
Study design, and data collection
This is a single-center retrospective cohort study. Between January 2013 and December 2014, 314 consecutive patients with ADHF were admitted to our institute. ADHF was diagnosed by clinical observation if a patient manifested S3 gallop and pulmonary rales or if alveolar and/or interstitial edema was present by chest radiograph based on the Framingham criteria [10]. We included 41patients (13.1%) who received TLV for this study population. The decision to prescribe TLV was entirely left to each attending physician. TLV was considered effective if adequate increase in urine output or body weight loss was obtained. Cases in which no sufficient change in urine volume or weight was observed were considered to be ineffective. Its initial and titration dose, and therapeutic duration were not uniformly arranged. Concomitant medications and adjunctive therapies including circulatory/ventilatory support were determined by the attending physician. We used TLV on a temporary basis during hospitalization to avoid prolonged administration. Among the study population, we identified 6 patients requiring outpatient TLV continuation (CONT group), whereas TLV was interrupted in 35 patients during hospitalization (INT group). The patient flow diagram is depicted in (Figure 1). Any baseline demographics and concurrent medications were confirmed from medical charts. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee.

Figure 1. Flow chart of study patients. ADHF, acute decompensated heart failure; TLV, Tolvaptan.
Statistical analysis
The continuous variables were expressed as the means ± SD or medians with 25th and 75th percentiles and the frequency as percentages for descriptive purposes. For continuous variables, comparisons between groups were performed by either Student’s t test or the Mann-Whitney U test. Frequency analysis was made using the χ2 test or Fisher exact test. All-cause death or rehospitalization for HF at 1 year was compared with Kaplan-Meier analysis and the log rank statistic between cases where TLV was effective and ineffective. The multiple logistic regression method was used to provide factors associated with TLV continuation and its subsequent outpatient maintenance as odds ratios (ORs) with 95% confidence intervals (CI). P<0.05 was considered significant, and all P values were 2-sided. Analyses were performed with PASW statistics version 18 (SPSS Inc., Chicago, IL).
Baseline characteristics are shown in Table 1. The proportion of the clinical scenario (CS) 2 and 3 tended to differ between groups, although this difference in clinical presentation was not statistically significant. There were significantly more patients in the CONT group who had history of at least ≥ 2 rehospitalization for HF than in the INT group. With respect to HF etiology, the CONT group had significantly more patients with dilated cardiomyopathy (DCM) than the INT group. More patients in the CONT group underwent open heart surgery including mitral valve surgery for functioning mitral regurgitation and coronary artery bypass grafting. Device therapies including pacemakers, implantable cardioverter defibrillators, and cardiac resynchronization therapy-defibrillators had been more frequently adopted in the CONT group. Diastolic blood pressure and heart rate on admission were lower in the CONT group than in the INT group, but were not statistically significant. Left ventricular function and renal function parameters tended to be worse in the CONT group than in the INT group. Hemoglobin levels were significantly lower in the CONT groups than those in the INT group. Serum sodium values did not differ between the groups. The number of patients with hypoalbuminemia was significantly higher in the INT group.
The status of TLV and other diuretics use, and concurrent baseline medications are summarized in Table 2. Initial TLV dosage was lower in the CONT group than the INT group, whereas the maximal titration dose was identical between the groups. Hypernatremia defined as serum sodium level ≥ 150 mEq/L following TLV administration was observed in 2 patients in the INT group. TLV was discontinued in 3 patients in the INT group because of adverse elevated serum sodium levels. With regard to concomitant medications, loop diuretics such as furosemide and azosemide were administered in all patients. Significantly more patients in the CONT group received thiazide diuretic than in the INT group. Pimobendan and amiodarone were more frequently administered in the CONT group than in the INT group, although this was not statistically significant.
Table 1.Baseline patient characteristics
| |
Total (n = 41) |
INT
(n = 35) |
CONT
(n = 6) |
p |
| Age mean, years |
73.5 ± 14.1 |
73.7 ±15.1 |
72.3 ± 9.4 |
0.824 |
| Body mass index, mean kg/m2 |
23.0 ± 4.1 |
23.2 ± 5.1 |
22.0 ± 1.9 |
0.317 |
| Body surface area, mean, m2 |
1.94 ± 0.34 |
1.94 ± 0.36 |
1.94 ± 0.22 |
0.983 |
| Male, n (%) |
19 (46.3) |
17 (48.6) |
2 (33.3) |
0.668 |
| NYHA Class III/IV, n (%) |
10 (24.3)/31 (75.7) |
8 (22.9)/27 (77.1) |
2 (33.3)/4 (66.7) |
0.621 |
| Clinical scenario, n (%) |
|
|
|
|
| 1 |
11 (26.9) |
10 (28.6) |
1 (16.7) |
1.000 |
| 2 |
11 (26.9) |
11 (31.4) |
0 |
0.167 |
| 3 |
14 (34.1) |
10 (28.6) |
4 (66.6) |
0.157 |
| 4 |
4 (9.7) |
3 (8.6) |
1 (16.7) |
0.432 |
| 5 |
1 (2.4) |
1 (2.8) |
0 |
1.000 |
| Prior HF hospitalization, n (%) |
17 (41.4) |
13 (37.1) |
4 (66.6) |
0.212 |
| Prior HF hospitalization ≥ 2, n (%) |
7 (17.1) |
3 (8.6) |
4 (66.6) |
0.005 |
| Etiology of HF |
|
|
|
|
| Acute coronary syndrome, n (%) |
5 (12.2) |
4 (11.4) |
1 (16.7) |
0.567 |
| Ischemic cardiomyopathy, n (%) |
9 (21.9) |
8 (22.9) |
1 (16.7) |
1.000 |
| Dilated cardiomyopathy, n (%) |
5 (12.2) |
2 (5.7) |
3 (50.0) |
0.017 |
| Valvular/structural n (%) |
6 (14.6) |
6 (17.1) |
0 |
0.567 |
| Others |
16 (39.1) |
15 (42.9) |
1 (16.7) |
0.376 |
| Prior PCI, n (%) |
8 (19.5) |
6 (17.1) |
2 (33.3) |
0.578 |
| Prior open heart surgery, n (%) |
6 (14.6) |
3 (8.6) |
3 (50.0) |
0.031 |
| Hypertension, n (%) |
23 (56.1) |
20 (57.1) |
3 (50.0) |
1.000 |
| Diabetes, n (%) |
24 (58.5) |
20 (57.1) |
4 (66.6) |
1.000 |
| Atrial fibrillation, n (%) |
18 (43.9) |
16 (45.7) |
2 (33.3) |
0.676 |
| Pacemaker/ICD/CRT-D, n (%) |
8 (19.5) |
4 (11.4) |
4 (66.6) |
0.009 |
| |
|
|
|
|
| Systolic BP, mmHg |
106.6 ± 19.0 |
107.2 ± 17.3 |
102.8 ± 19.3 |
0.730 |
| Diastolic BP, mmHg |
61.6 ± 12.2 |
63.0 ± 12.1 |
53.6 ± 10.8 |
0.084 |
| Heart rate, beats/min |
81.9 ± 16.8 |
83.5 ± 16.5 |
72.1 ± 16.3 |
0.190 |
| LVEF, % |
39.1 ± 19.4 |
39.1 ± 20.0 |
32.5 ± 21.7 |
0.462 |
| LVEF ≤ 30%, n (%) |
15 (36.6) |
11 (31.4) |
4 (66.7) |
0.168 |
| BNP, pg/ml |
895.1 [521.6, 2000.0] |
804.7 [462.6, 1795.9] |
919.9 [658.9, 2000.0] |
0.447 |
| Hemoglobin, g/dl |
11.71 ± 2.21 |
11.86 ± 2.36 |
10.83 ± 0.53 |
0.029 |
| Serum BUN, mg/dl |
36.99 ± 21.43 |
34.88 ± 18.53 |
49.28 ± 33.53 |
0.439 |
| Serum creatinine, mg/dl |
1.63 ± 1.17 |
1.53 ± 1.10 |
2.19 ±1.47 |
0.310 |
| CrCl, ml/min |
41.54 ± 28.25 |
43.12 ± 29.31 |
32.31 ± 20.52 |
0.302 |
| CrCl ≤ 30 ml/min, n (%) |
16 (39.1) |
12 (34.3) |
4 (66.7) |
0.187 |
| Serum Na, mEq/l |
134.5 ± 5.4 |
134.5 ± 5.7 |
134.3 ± 3.5 |
0.670 |
| Serum Na ≤ 134 mEq/l |
15 (36.6) |
13 (37.1) |
2 (33.3) |
1.000 |
| Serum albumin, g/dl |
3.25 ± 0.64 |
3.16 ± 0.65 |
3.73 ± 0.31 |
0.033 |
BP: blood pressure; CrCl: creatinine clearance; CRT: cardiac resynchronization therapy-defibrillator; HF: heart failure; ICD: implantable cardioverter defibrillator; LV: left ventricular ejection fraction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; Continuous variables are expressed as mean ± SD or median [interquartile range].
Table 2. Tolvapan usage and baseline concomitant medications
| |
Total (n = 41) |
INT
(n = 35) |
CONT
(n = 6) |
p |
| <Tolvaptan usage> |
|
|
|
|
| Initial dose median, mg |
7.50 [7.50, 7.50] |
7.50 [7.50, 7.50] |
5.62 [3.75, 7.50] |
0.065 |
| Maximal dose median, mg |
7.50 [7.50, 15.00] |
7.50 [7.50, 15.00] |
11.25[3.75, 15.00] |
0.871 |
| Time to start after admission, days |
6 [3, 11] |
6 [3, 11] |
4 [1, 9] |
0.448 |
| Duration of therapy, days |
9 [4, 23] |
9 [4, 21] |
9 [7, 35] |
0.605 |
| Effective, n (%) |
26 (63.4) |
20 (57.1) |
6 (100) |
- |
| Ineffective, n (%) |
15 (36.6) |
15 (42.9) |
0 |
- |
| Peak serum Na ≥ 150 mEq/l, n (%) |
2 (4.9) |
2 (5.7) |
0 |
1.000 |
| Unscheduled discontinuation, n (%) |
3 (7.3) |
3 (8.6) |
0 |
1.000 |
| <Concomitant medications> |
|
|
|
|
| Furosemide, n (%) |
36 (87.8) |
31 (88.6) |
5 (83.3) |
0.567 |
| Dose, mg |
60 [40, 80] |
60 [40, 80] |
60 [30, 80.] |
0.888 |
| Azosemide, n (%) |
10 (24.4) |
9 (25.7) |
1 (16.7) |
1.000 |
| Dose, mg |
60 [53, 75] |
60 [45, 90] |
60 [60, 60] |
0.852 |
| Trichlormethiazide, n (%) |
16 (39.1) |
11 (31.4) |
5 (83.3) |
0.026 |
| Dose, mg |
1.50 [1.00, 2.00] |
1.00 [1.00, 2.00] |
2.00 [1.00, 2.00] |
0.584 |
| Inotropes, n (%) |
19 (46.3) |
17 (48.5) |
2 (33.3) |
0.668 |
| Carperitide, n (%) |
8 (19.5) |
8 (22.8) |
0 |
0.323 |
| ACE-inhibitors/ARB, n (%) |
24 (58.5) |
20 (57.1) |
4 (66.7) |
1.000 |
| β-blockers, n (%) |
24 (58.5) |
19 (54.2) |
5 (83.3) |
0.373 |
| Aldosterone blockers, n (%) |
14 (34.1) |
12 (34.2) |
2 (33.3) |
1.000 |
| Digoxin, n (%) |
10 (24.3) |
9 (25.7) |
1 (16.7) |
1.000 |
| Pimobendan, n (%) |
5 (12.2) |
3 (8.5) |
2 (33.3) |
0.148 |
| Amiodarone, n (%) |
5 (12.2) |
3 (8.5) |
2 (33.3) |
0.148 |
ACE: angiotensin converting enzyme; ARB: angiotensin II receptor blocker. Continuous variables are expressed as median [interquartile range].
The Kaplan-Meier estimate of the event rate for death of any cause or HF rehospitalization at 1 year was 47.5% in cases where TLV was effective compared with 67.9% where TLV was ineffective (log rank p =0.046, Figure 2).
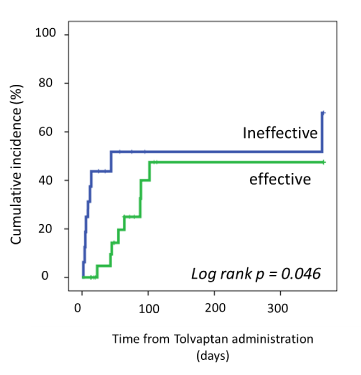
Figure 2. Cumulative incidence of all-cause death or rehospitalization for heart failure at 1 year between Tolvaptan effective and ineffective cases. Probability values were calculated with log-rank test.
The logistic regression method was used to investigate whether any possible correlations exist between the baseline demographics and outpatient TLV continuation (Table 3). Univariate modeling showed ≥ 2 hospitalizations for recurrent HF (OR, 20.66; 95% CI, 2.60 – 163.80; p=0.001), co-administration of thiazide diuretic (OR, 10.90; 95% CI, 1.13 – 104.80; p=0.016), and the use of any device therapies (OR, 15.50; 95%CI, 2.11 – 113.54; p=0.002) were correlates of outpatient TLV use, whereas multivariate modeling demonstrated only ≥ 2 hospitalizations for recurrent HF was associated with outpatient TLV continuation (OR, 24.55; 95% CI, 1.96 – 306.19; p=0.013) (Table 3).
Table 3. Logistic regression analysis showing the predictor of outpatient Tolvaptan continuation
| |
Univariate |
Multivariate |
| Variables |
OR |
95% CI |
p value |
OR |
95% CI |
p value |
| Age ≥ 75yrs |
0.94 |
0.16-5.33 |
0.948 |
|
|
|
| Gender (male) |
0.52 |
0.08-3.27 |
0.489 |
|
|
|
| Body mass index ≥ 22.5 kg/m2 |
2.11 |
0.34-13.09 |
0.413 |
|
|
|
| Body surface area ≥ 1.9 m2 |
1.68 |
0.27-10.42 |
0.572 |
|
|
|
| Clinical scenario 3 on admission |
5.00 |
0.78-31.76 |
0.069 |
|
|
|
| Prior HF hospitalization ≥ 2 |
20.66 |
2.60-163.80 |
0.001 |
24.55 |
1.96-306.19 |
0.013 |
| Diabetes |
1.50 |
0.24-9.30 |
0.662 |
|
|
|
| Ischemic cardiomyopathy |
0.67 |
0.06-6.64 |
0.735 |
|
|
|
| Atrial fibrillation |
0.59 |
0.09-3.67 |
0.572 |
|
|
|
| LVEF ≤ 30% |
4.36 |
0.49-27.51 |
0.098 |
|
|
|
| Sodium ≤ 134 mEq/l |
0.84 |
0.13-5.27 |
0.858 |
|
|
|
| CrCl < 30 ml/min |
3.83 |
0.61-24.02 |
0.133 |
|
|
|
| Furosemide ≥ 60 mg |
1.18 |
0.21-6.71 |
0.846 |
|
|
|
| Thiazide |
10.90 |
1.13-104.80 |
0.016 |
14.40 |
0.94-220.54 |
0.055 |
| Pimobendan |
5.33 |
0.67-42.23 |
0.087 |
|
|
|
| Device therapy |
15.50 | 2021 Copyright OAT. All rights reserv
2.11-113.54 |
0.002 |
|
|
|
CrCl: creatinine clearance; HF: heart failure; LVEF: left ventricular ejection fraction
The major findings of the present study are summarized as follows: (1) outpatient TLV use was required in refractory HF hospitalized patients with a greater chance of HF exacerbation, requirement for open heart surgery, device therapy, lower hemoglobin levels, and combined thiazide diuretic use; (2) death of any cause or rehospitalization for HF at 1 year was significantly higher in patients in whom TLV was considered to be ineffective; and (3) multivariate analysis demonstrated a correlation between multiple HF exacerbations ≥ 2 times and outpatient TLV continuation. Similar to the high prevalence of device therapy or combined thiazide use in the CONT group, the number of prior rehospitalizations may be a marker of increased disease severity of HF. Higher prevalence of prior open heart surgery should also reflect the advanced-stage HF in the CONT group, but conversely, an element of postsurgical cardiac constriction possibly made fluid management more difficult only with the use of conventional diuretics. Our results suggest that outpatient TLV use may be necessary for patients experiencing repeated worsening of refractory HF.
Management of ADHF is yet challenging due to the heterogeneity in disease etiology and absence of robust evidence-based guidelines [11]. The majority of patients with congestion appear to respond well to initial therapies with loop diuretics [12]. However, insufficient decongestion is more common than previously understood and results in longer hospital stays, early exacerbations, and increased mortality [3]. Adequate decongestion is sometimes unachievable particularly in patients with refractory HF, most of whom are classified as stage D [13] and resistant to poly-pharmaceuticals including multiple diuretics. Moreover, conventional diuretics such as loop and thiazide diuretics have several potentially detrimental effects on renal function [3,14] and poor response to diuretics frequently occurs in hospitalized patients with HF despite escalating the diuretic dose [4]. Current guidelines provide only nonspecific guidance in diuretic regimen design for patients with diuretic resistance [15].
Since 2010 TLV has been indicated to treat congestive HF resistant to conventional diuretic use in Japan [8] and has been reported to improve quality of life and avert WRF in hospitalized patients with HF [5,7]. Recent studies indicate beneficial aspects of earlier TLV administration in ADHF in terms of preserved renal function and shorter hospital stays [16,17]. However, there is a concern about universal or prolonged TLV use in HF management because little is available on beneficial impact on long-term mortality or HF–related morbidity [9]. In addition, not much has been done to clarify the cost effectiveness of aggressive TLV use.
To date, accepted definition of diuretic resistance has not been described because no validated metric of diuretic response has been established [14]. Furthermore, it is often difficult to identify diuretic resistance due to chronic loop diuretic use. Therefore, optimal strategy to utilize TLV in diuretic-resistant patients with HF will inevitably be controversial at this time. A stepped pharmacological approach has been proposed to treat diuretic-resistant patients with HF [14]. According to the approach, TLV is regarded as a final step alternative following combined conventional diuretic therapy [14]. The present data obtained from a single center, where TLV was conservatively used, may represent the way of TLV use as a last resort for a special population of patients with refractory HF, who are administered combined loop and thiazide diuretics. Nevertheless, the abovementioned stepped approach [14] is an expert opinion and may reflect medical environments where TLV use is not reimbursed for HF in contrast to that of Japan.
In terms of the short-term outcome, the timing of TLV initiation may have affected our results. Prompt administration of TLV has been reported to preserve renal function and improve survival of patients with ADHF hospitalized in intensive care units [6], while few patients in our study population received TLV from the first day of admission.
Moreover, from a long-term perspective, a more proactive approach with early TLV introduction before repeated exacerbations could have positively changed the clinical course of our study patients. The currently proposed AHA/ACC staging classification [13] emphasizes the importance of early detection and intervention in symptomatic HF because the recurring intermittent exacerbations progressively result in end stage where the major focus of care is palliation [18]. TLV therapy at the initial phase of HF management before prolonged exposure to loop diuretics can potentially prevent diuretic resistance and improve subsequent long-term clinical outcome.
In this study, significantly poorer immediate-term clinical outcomes in TLV ineffective cases were noted as compared with those in TLV effective cases. In the light of the appropriate use of this novel aquaretic vasopressin antagonist, several measures have been explored to successfully identify potential TLV responders [19,21]. Imamura et al. showed that the proportion of potential non-responders to TLV is 30% in hospitalized patients with ADHF, and higher age and impaired renal function are associated with poor response to TLV [19,22]. The proportion of ineffective cases was 36% in our population, consistent with the results of the study by Imamura [22]. With regard to baseline renal function, patients with renal insufficiency were more prevalent in our study population as compared to other reports [6, 17]. In an aging population (83.4 ± 9.6 years), TLV administration during the acute phase has been reported to prevent WRF and improve survival in hospitalized patients with ADHF [17]. However, data from the TACT-ADHF study were obtained from patients with relatively preserved renal function [17]. In current status of aging world, the number of older patients with HF and less preserved renal function will increase further in the future [23]. Favorable conditions must be created for TLV to exert an intended effect in elderly patients with advanced renal dysfunction, which is one of the most challenging clinical settings of HF.
The present study has several limitations. First, this was a single-center retrospective study. None of the initial and titration doses, and therapeutic duration of TLV was prespecified. Furthermore, we allowed wide variation in the therapeutic regimens of other concurrent medications. Second, the study population was small. Because no power or sample size calculation was performed, the possibility of a type II error should be considered. Third, we did not assess any potential measure to determine the TLV responsiveness such as urine osmolality and urine aquaporin-2 [19,21]. Therefore, we may have missed potential responders to TLV among TLV ineffective cases. Finally, the difference of DCM between the INT and the CONT groups may be accounted for by a selection bias. Because this diagnosis is eligible for public health benefits in Japan, patients with DCM do not require paying additional treatment costs for outpatient TLV use.
In conclusion, outpatient TLV use may be necessary for patients with refractory HF who experience multiple exacerbations. Even with conservative use, patients who require TLV on an outpatient basis can exist in real world. Further dedicated studies are needed to optimize TLV therapy in terms of its initiation timing, its dose, and its duration.
- Group JCSJW. Guidelines for treatment of acute heart failure (JCS 2011). Circulation journal : official journal of the Japanese Circulation Society 77: 2157-2201. [Crossref]
- Metra M, Cleland JG, Weatherley BD, Dittrich HC, Givertz MM, et al. (2010) Dyspnoea in patients with acute heart failure: an analysis of its clinical course, determinants, and relationship to 60-day outcomes in the PROTECT pilot study. European journal of heart failure 12: 499-507. [Crossref]
- Felker GM and Mentz RJ (2012) Diuretics and ultrafiltration in acute decompensated heart failure. Journal of the American College of Cardiology 59: 2145-2153. [Crossref]
- Ellison DH (2001) Diuretic therapy and resistance in congestive heart failure. Cardiology 96:132-43.
- Gheorghiade M, Konstam MA, Burnett JC, Jr Grinfeld L, Maggioni AP, et al. (2007) Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. Jama 297: 1332-1343. [Crossref]
- Shirakabe A, Hata N, Yamamoto M, Kobayashi N, Shinada T, et al. (2014 Immediate administration of tolvaptan prevents the exacerbation of acute kidney injury and improves the mid-term prognosis of patients with severely decompensated acute heart failure. Circulation journal : official journal of the Japanese Circulation Society 78: 911-921.
- Imamura T, Kinugawa K (2015) Mid-Term Administration of Tolvaptan Improves Renal Function Accompanied by Dose-Reduction in Furosemide in Aquaporin-Defined Responders. International heart journal 56: 686-687. [Crossref]
- Matsuzaki M, Hori M, Izumi T, Fukunami M, Tolvaptan I (2011) Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy 25: S33-45. [Crossref]
- Konstam MA, Gheorghiade M, Burnett JC, Jr., Grinfeld L, Maggioni AP, et al. (2007) Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan I. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. Jama 297: 1319-1331. [Crossref]
- McKee PA, Castelli WP, McNamara PM, Kannel WB (1971)The natural history of congestive heart failure: the Framingham study. The New England journal of medicine 285: 1441-1446. [Crossref]
- Gheorghiade M, Pang PS (2009) Acute heart failure syndromes. Journal of the American College of Cardiology 53: 557-573.
- Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Committee ASA and Investigators (2006) Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. Journal of the American College of Cardiology 47: 76-84. [Crossref]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, et al. (2005) ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 112: e154-235.
- Ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, et al. (2015) Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nature reviews Cardiology 12: 184-192. [Crossref]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. (2013)ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: 1810-1852. [Crossref]
- Matsukawa R, Kubota T, Okabe M and Yamamoto Y (2015) Early use of V2 receptor antagonists is associated with a shorter hospital stay and reduction in in-hospital death in patients with decompensated heart failure. Heart and vessels. [Crossref]
- Kimura K, Momose T, Hasegawa T, Morita T, Misawa T, et al. (2015) Early administration of tolvaptan preserves renal function in elderly patients with acute decompensated heart failure. Journal of cardiology. [Crossref]
- Goodlin SJ (2009) Palliative care in congestive heart failure. Journal of the American College of Cardiology 54: 386-396.
- Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, et al. (2013) Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients--association between non-responders and chronic kidney disease. Circulation journal : official journal of the Japanese Circulation Society 77: 397-404. [Crossref]
- Imamura T, Kinugawa K, Minatsuki S, Muraoka H, Kato N, et al. (2013) Urine osmolality estimated using urine urea nitrogen, sodium and creatinine can effectively predict response to tolvaptan in decompensated heart failure patients. Circulation journal : official journal of the Japanese Circulation Society 77: 1208-1213.
- Imamura T, Kinugawa K, Fujino T, Inaba T, Maki H, et al. (2014) Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circulation journal: official journal of the Japanese Circulation Society 78: 2240-2249.
- Imamura T, Kinugawa K, Minatsuki S, Muraoka H, Kato N, et al. (2013) Tolvaptan can improve clinical course in responders. International heart journal 54: 377-381. [Crossref]
- Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, et al (2010) Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. Jama 303: 2141-2147. [Crossref]


