Abstract
Objective
Assess the impact of ovarian torsion on histology. We also investigate the impact of clinical and biochemical factors on histological outcomes following ovarian torsion.
Design
Retrospective analysis of prospectively maintained clinical data.
Setting
St. Thomas’ hospital (1999-2010).
Population
72 patients with confirmed ovarian torsion at surgery were analysed with histology analysis in 67/72 (93%). Complete clinical, biochemical and pathology data in 58/72 (80.5%).
Methods
Reanalysis of all histological samples in the context of clinical and biochemical data.
Main Outcome measure
Two novel scoring systems Oocyte Preservation Score (OPS) and Tissue Necrosis Score (TNS)) were developed to evaluate the effect of torsion. Low OPS represents severe inflammation and necrosis whilst a high OPS represents a mildly affected ovary. TNS represents percentage necrosis for each specimen. Post-Menopausal Torsions (PMT) were evaluated by ovarian stromal appearance and inflammatory pattern. Clinical and biochemical factors were assessed as predictors of ovarian histological preservation utilising multiple regression modelling.
Results
The mean age was 38 years (range 17 to 86 years). There was a known menstrual history in 54 patients; 44/54 (81.4%) were premenopausal and of these 25/44 (56.8%) were nulliparous with mean age 26 years (range 17 to 39 years). 5/44 (11.3%) were pregnant and 10/54 (18.5%) were post-menopausal. 17/67 (25.4%) of the patients had dermoid cysts, with 23/67 (34.3%) having no co-existing cystic pathology. 1 patient had a malignant granulosa-cell tumour.
Older women over the age of 52 years were more likely to have suspected malignancy 12/67 (17.9%) and lower OPS (p < 0.0001). Clinical predictors of torsion were associated with low OPS; pyrexia >38°C (R2 = 0.28, P = 0.05), leucocytosis >14.9 (R2 = 0.32, P = 0.001); CRP > 75 (R2 = 0.19, P = 0.013).
Conclusions
TNS and OPS were developed to evaluate histopathological changes of torsion. Fever, leukocytosis, CRP and prolonged torsion (>70 hrs) were associated with lower OPS. Preserved oocyte follicles were observed in 42/67 (62.6%) of cases, implying preserved ovarian function in the vast majority of patients who had oophorectomy for ovarian torsion.
Key words
ovary, pregnancy, ovarian function, ovarian torsion, detorsion
Introduction
Adnexal torsion can occur at any age, from intrauterine life to post-menopausal [1,2]. The incidence in the paediatric population is 0.02% [3]. It is reported to be 2.7% of all gynaecological emergencies [4]. Pre-operative diagnosis is often challenging and the treatment involves either detorsion or resection of torted adnexae. Adnexal torsion usually affects the ovary and fallopian tube together. Isolated tubal torsion can occur, particularly in pathological tubes [5]. Even though enlarged cystic ovaries are most likely to tort, torsion can occur in previously normal ovaries [6].
Clinical studies on ovarian detorsion have shown >91% follicular development rate at ultrasound follow-up studies [7,8]. An animal study looked at the macroscopic and histological appearances of rodent ovaries after surgically induced torsion at controlled periods (ranging 4 to 36 hours). At 36 hours there was a watershed, where ovaries displayed irreversible infarction [9]. Lesser durations of torsion induced only minor reversible ischaemic changes. Macroscopically, ovaries were similarly blue-black in all cases [9]. A watershed time for ovarian preservation after torsion has not been demonstrated in humans. Duration of torsion has been proposed to be a possible predictor for histological preservation [6]. At present there is no clinical data to predict which ovaries are viable at surgery after ovarian torsion. Oophorectomy has an impact on subfertility in these patients who are usually of child bearing age. We look at the impact of various clinical and biochemical predictors in the patients presenting with torsion on ovarian preservation at histology. We describe two novel histological scoring systems to assess ovarian viability.
Methods
All patients with ovarian torsion were identified by electronic searches of clinical coding/electronic databases, radiology, and pathology reports. All ovarian pathology reports stored in the department archive where checked by PM to find all the cases of ovarian torsion for our study period. Pathology reports of ovarian resections carried out for suspected torsions over eleven-years were reviewed to confirm histological evidence of torsion. The tissue sections were retrieved from the Pathology Laboratory archives of St Thomas’ Hospital and reviewed independently by two Consultant Pathologists (AP and PM), who have specialist interest in gynaecological pathology. At the time of review, both pathologists were blinded to the clinical parameters. The clinical notes were obtained from notes and electronic records systems (WinDIP, Civica, UK). The data was collected on an Excel spreadsheet, and analysed on SPSS v20 (IBM, Inc. US).
Development of the histological scoring system
All histology was retrieved for ovarian cystectomies and oophorectomies, performed for torsion from 1999 to 2010. The first pathologist (PM) reviewed the tissue sections (stained with Haematoxylin and Eosin) to confirm original pathological diagnosis and to assess the extent of tissue necrosis and oocyte preservation. One to thirteen 3 µm-sections taken through the whole ovarian sample were analysed per patient. Each patient had a mean of 6 sections [1-14] reviewed for the development of the score. Laterality of tortion and macroscopic appearances including size noted at the time of specimen sampling were recorded. Other histological parameters recorded at the time of review included: presence/absence of oedema, congestion, haemorrhage and necrosis, with/without inflammation; type of inflammation (neutrophil-rich or macrophage-predominant in an effort to correlate with the duration of torsion), and presence/absence of surface exudates.
The extent of overall necrosis within each ovary was quantified by evaluating the percentage necrosis (TNS) for each specimen. TNS is a visual assessment of percentage necrosis. TNS was determined for all specimens Therefore, every patient had data on TNS-minimum (least affected specimen), TNS-maximum and TNS-mean. TNS-mean is the average for all slides, evaluated for each patient. Furthermore, we quantified necrosis according to ovarian anatomical location (TNS-medullary/cortical). TNS-cortical and TNS-medullary was determined in each of the 1-14 sections per ovarian sample per patient. A mean for each (TNS-cortical/medullary) was calculated per patient.
An OPS was developed to encompass both the structural preservation of the follicles and the integrity of their surrounding microenvironment. Oocyte follicle preservation was assessed in a qualitative and semi-quantitative manner. The distinction between preserved and necrotic ovarian follicles was based on the histological cellular integrity (cytoarchitecture). The pattern of severity progression (determined by degree of inflammatory changes and tissue necrosis) were qualitatively noted by PM after examining all the specimens. In post-menopausal women, where oocyte follicles are naturally depleted, the sub-cortical germinal layer was evaluated for histological integrity and inflammatory features.
An initial statistical analysis was performed comparing the binary outcome histology (presence/absence inflammation and necrosis), with clinical data (e.g. duration of torsion). The variables that yielded statistically significant results were kept in the score whilst the remainder were discarded. Ischaemic changes to the ovary present a continuous and variable process with many gradations of increasing inflammation and progressive necrosis. OPS 4, 6, 8 and 10 were chosen to reflect the range of ischaemic changes (Figure 1).
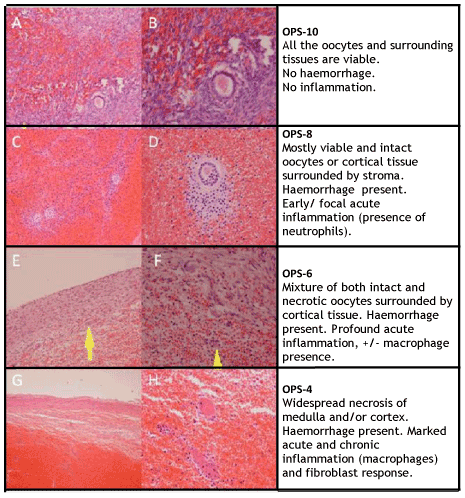
Figure 1. Oocyte Preservation Score histological appearance. (A) and (B) show the appearance of OPS 10 at x100 and x200 magnification, respectively. Note the focal haemorrhagic appearance, and a preserved oocyte follicle is surrounded with normal ovarian stroma. (C) and (D) show the appearance of OPS 8 at x100 and x200 magnification, respectively. Note diffuse haemorrhage in medulla. Preserved oocytes are still surrounded by some normal stroma. (E) and (F) show the appearance of OPS 6 at x100 and x200 magnification, respectively. Note a mixture of both viable and necrotic oocytes, with the presence of acute inflammation (neutrophils). (G) and (H) show the appearance of OPS 4 at x100 and x200 magnification, respectively. There is widespread necrosis of both medulla and cortex, with presence of chronic inflammation (macrophages).
PM reviewed the specimens again after finalising the scoring system, to ascertain the scores given to all specimens.
The second pathologist (AP) applied the OPS system to re-score all specimens, blinded to all other data. The cases with differing OPS scores were reviewed at a later time and a consensus was reached. The OPS was subsequently applied to the clinical data for final analysis.
Inclusion criteria
We included all cases that were surgically proven to be adnexal torsions.
Statistical analysis
Continuous data are presented as mean with Standard Deviation (SD), Odds Ratio (OR) and 95% confidence intervals (95%CI); qualitative results are presented as a distribution of a number of patients. For multivariable analysis, logistic linear regression was performed to determine the most significant variables influencing the outcome of interest before carrying out multivariable analysis. The alpha value of 0.05 was determined as significant at a 95%CI. Pearson’s correlation test was used to assess agreement in continuous data. The concordance between the two pathologist OPS data sets was analysed by the Cohen’s Kappa-test statistic. All analyses were performed in SPSS v20 (IBM, Inc. US).
Ethical approval
The study was considered by the Chair, King’s College Hospital Research Ethics Committee, National Research Ethics Service, who advised that the project did not require ethical review under the terms of the Governance Arrangements for Research Ethics Committees in the UK 08/01/2009.
Results
Demographics and patient population
72 patients with adnexal torsion were identified. 69/72 (98.5%) pathology specimens were found for adnexal torsion; 2 were isolated tubal torsions. 67/72 (93%) patients had ovarian histology specimens available. 58/72 (80.5%) had both clinical and pathology data available.
The mean age at ovarian torsion was 38 years (17-86 years). Data on the last menstrual period was available in 54/58 (93.1%). 44/54 (81.5%) were pre-menopausal, whilst 10/54 (18.5%) were post-menopausal. 38/54 (70.3%) had a current menstrual record. 22/38 (57.8%) were on cycle day 1-11. 16/38 (42.1%) were on day 12-34. 5/54 (9.3%) were pregnant (gestations: 7, 13, 16, 20 and 34wk). 5/54 (9.3%) had a coil. 6/54 (11.1%) had incomplete data.
Pre-menopausal non-pregnant group: 25/44 (56.8%) nulliparous, 12/44 (27.2%) multiparous; 2/44 (4.5%) parity not recorded. Pregnant group: 4-multigravidae, 1-primigravidae. Pre-menopausal nulliparous women had mean age of 27 years (range 17 to 39 years).
While 3/58 (5%) women had previous deliveries by caesarean section, 27/58 (46.5%) had vaginal deliveries prior to torsion. 14/58 (24%) had previous abdominal surgery. 12/58 (21%) had previously known ovarian cysts.
33/58 (56.9%) had sudden onset pain. 16/58 (27.5%) had gradual pain build-up. 36/58 (69.1%) had severe pain, 33/58 (56.9%) requiring opiates (WHO-4). 11/58 (19%) WHO-3, 3/58 (5%) WHO-2, 2/58 (3%) WHO-1, 3/58 (5%) None. 47/58 (81%) had nausea and 38/58 (65%) had vomiting.
3 patients (ages 23, 24 and 28 years) had a recurrence of ovarian torsion. Two had recurrence of torsion in a previously detorted ovary after 1.5 yr and 11 yrs. These patients were treated by oophorectomy. The third patient had an oophorectomy for torsion in childhood and had ovarian torsion associated with a dermoid cyst in the remaining ovary. She was treated with detorsion and concurrent ovarian cystectomy. OPS of these patients were 6, 8 and 8.
Ultrasound reports were reviewed in 53/72 (74%). Ultrasound indicated pathology (e.g. ovarian cysts of any size, including simple cysts) in 45/53 (85%). 13/53 (24.5%) had a significant ovarian lesion (>5 cm, non-simple). Ultrasound was not suggestive of ovarian torsion in 32/53 (60%). Ovarian torsion was specifically mentioned in the report in 8/53 (15%).
Operative data
45/58 (78%) were treated by oophorectomy. 13/58 (22%) were treated by detorsion and concurrent cystectomy. 20/58 (34%) were treated laparoscopicaly. Total laparotomy rate was 38/58 (65.5%).
Pathology
69/72 (96%) had histology available for review. Adnexal torsion including the ovary occurred in 67/69 (97%); isolated tubal torsion 2/69 (3%) (Table 1) Pathology data.
Table 1. Pathology data. *Dermoid cyst (mature cystic teratoma). Simple cyst group includes 1 corpus leteum in early pregnancy, and a small follicular cyst.
|
Type of pathology
|
Number of patients (n=69)
|
|
Ovarian torsion
Tubal torsion
|
67/69 (97.1%)
2/69 (2.9%)
|
|
No ovarian cystic pathology
|
22/67 (32.8%)
|
|
Dermoid cyst*
|
17/67 (25.4%)
|
|
Simple cyst
|
9/67 (13.4%) (2 physiological)
|
|
Serous cystadenoma
|
9/67 (13.4%)
|
|
Mucinous cystadenoma
|
6/67 (9.0%)
|
|
Struma ovarii
|
2/67 (3.0%)
|
|
Thecofibroma
|
1/67 (1.5%)
|
|
Adult cell granulosatumour
(borderline tumour)
|
1/67 (1.5%)
|
|
Serous cystadenofibroma
|
1/67 (1.5%)
|
|
Endometriotic cyst
|
1/67 (1.5%)
|
Two consultant pathologists blinded to clinical results reviewed the sections independently. The k-coefficient for the full 4-tier OPS was 0.6. Most disagreement occurred for OPS-4 and OPS-6 (Figure 1). In OPS-6, a small number of preserved follicles were seen, with profuse acute inflammation and early chronic inflammation. In OPS-4, a very small number of preserved follicles were seen with profound chronic inflammation. The k-coefficient for a 2-tier score, Low (OPS-4&6)/High (OPS-8&10), was 0.73.
Co-existent cystic pathology was present in 45/67 (67%). See Table 3. 22/67 (33%) ovarian torsion had no other identifiable pathology. 1/67 (1.5%) ovary contained a malignancy (granulosa-cell tumour). This patient had further surgery at a later date. However, 11/67 (16%) patients underwent a malignancy staging procedure involving a total abdominal hysterectomy, bilateral salpingo-oophorectomy and omentectomy, of whom none had malignancy. This was based on pre-operative imaging findings, and overall risk assessment for malignancy.
A dermoid cyst was co-existent with a serous cystadenoma in one patient, and an endometrioma in another. Pathology occurring at corresponding gestations included: 34 weeks- cystadenoma; 20 weeks- dermoid cyst; 16 weeks- mucinous cystadenoma; 13 weeks- serous cystadenoma; 7 weeks- physiological follicular cyst. Fibrinous exudates (histological evidence of peritonitis) was absent in 46/69 (67%).
Oocyte preservation score (OPS)
OPS score was determined in all available cases (n = 67). The average overall OPS was 6.6. 25/67 (37%) had OPS-4. 15/67 (22%) had OPS-6. 8/67 (11.9%) had OPS-8. 19/67 (28.3%) had OPS-10.
Clinical variables that might influence the OPS included in the statistical model were: pyrexia, vomiting, analgesia requirement, CRP, leukocytosis, and duration of torsion. Using linear logistic regression modelling, the ‘best fit’ was determined for the most significant variables that might influence OPS. The three variables that demonstrated a significant correlation to OPS were pyrexia (P = 0.005, 95%CI ± 0.009, R2 = 0.28), WCC>14.9 (P = 0.001, 95%CI ± 0.155, R2 = 0.32) and CRP>75 (p = 0.013, 95%CI ± 0.739, R2 = 0.19). Clinical features that were included in the multiple regression analysis model, but were not statistically significant included age, vomiting, pain severity, analgesia requirement, leucorrhoea, and menorrhagia.
Duration of torsion was calculated from the clinical data. There was a wide range of time intervals. The shortest was under an hour from onset of pain to operation. In this case, it was a recurrence of torsion and the patient was confident of the diagnosis on presenting to casualty. The longest case was estimated to be 12000 hrs. This patient was seen acutely twice and discharged with analgesia. A laparoscopy was performed at a later date electively.
OPS-4&6, are more likely to be associated with >70 hrs ischaemia (Pearson’s correlation test; P = 0.02, R2 = 0.73). Prolonged torsion (>70 hrs) had significantly worse TNS-cortical (P = 0.03). OPS-10 contained a wide range of durations (range 7-12000 hrs; median = 72 hrs).
OPS during pregnancy included at: 7 weeks OPS-10; 13 weeks OPS-6; 16 weeks OPS-8; 20 weeks and 34 weeks OPS-4.
Tissue Necrosis Score (TNS)
TNS data followed a normal distribution. TNS-mean was 52%; median 56%; range (0-98%). 21% (12/58) had <10% TNS (and OPS-10). 10% (6/58) had >90% TNS (and OPS-4).OPS and TNS-mean also significantly correlated (R2 = 0.84, P = 0.001 )(Figure 2) correlates TNS-minimum and maximum with OPS.
2021 Copyright OAT. All rights reserv
The ovarian cortex showed resistance to ischaemia, being the last part of the ovary to be affected in each case. Therefore, the degree of cortical necrosis was found to correlate with the degree of follicular preservation (Figure 3). As the ovarian follicles are located in the ovarian subcortical area, they are the last structures to undergo infarction. OPS and TNS-cortical were significantly positively correlated (R2 = 0.90, P < 0.0001).
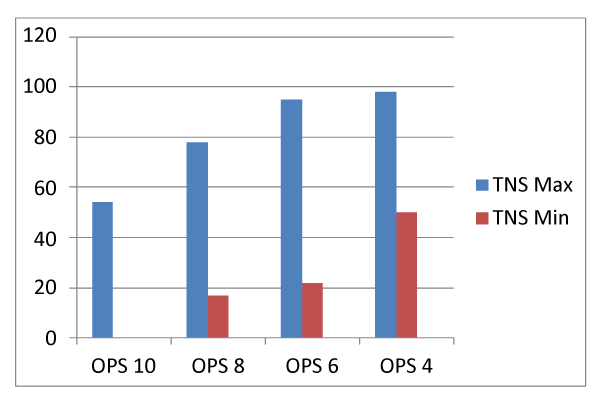
Figure 2. TNS-maximum and TNS-minimum correlated with OPS.
OPS and operative data
The oophorectomy rate was 78% (45/58), of which 67% had OPS 6&4. 12% had OPS-8, and 27% had OPS-10.
12/67 (18%) had a staging procedure for suspected but not proven ovarian cancer electively. These patients were older mean age 52 years compared to rest of the cohort, with significantly lower OPS (P < 0.0001). 7/10 (70%) of post-menopausal patients had OPS-4.
Malignancy was more likely to be suspected in older women due to the ultrasound appearance of the ovarian cysts. Markers of suspicion at ultrasonography included multi-loculated nature of cyst, thick septations, haemorrhagic contents in the cyst, and avascularity. One patient had raised tumour markers (CA125 = 239 U/ml, CA19-9 = 30 U/ml, CEA = 6.1 U/ml), and an avascular area seen within the ovarian tumour (fibrothecoma) on ultrasonography and CT-scan.
One pre-menopausal patient presented with a granulosa-cell tumour. Malignancy was not suspected pre-operatively therefore she had further surgery, after the oophorectomy.
OPS-10 (n = 19)
10/19 (53%) with OPS-10 required WHO-4 analgesia (opiates). The rest requiring: 3/19 (16%) no analgesia; 1/19 (5%) WHO-1; 2/19 (10.5%) WHO-2; 3/19 (16%) WHO-3.
11/19 (58%) were treated with oophorectomy; 5/19 (26%) with detorsion with cystectomy; 3/19 (16%) had no operative data available. There was no sign of cortical necrosis (TNS-cortical) in 15/19 (79%); 2/19 (10.5%) had 10% TNS-cortical; 2/19 (10.5%) had 20% TNS-cortical.
3/19 (16%) had raised CRP (73-256); 11/19 (58%) had low CRP (<5-27); 5/19 (26%) not done/recorded.
Discussion
We developed and validated two novel histological grading tools: OPS and TNS. This data informs us on the impact of ischaemia upon ovarian tissue, and likely clinical outcome of conservative management. It is also possible to use this grading system on ovarian biopsy or ovarian cystectomy tissue at the time of detorsion, in order to provide pathology diagnosis and individualised prognostic fertility information to pre-menopausal patients. Further research would be needed to ascertain the clinical application of ovarian biopsy in this context.
Medullary changes, including venous engorgement, oedema, and secondary acute inflammation are part of the immediate response in torsion acutely. Venous engorgement makes ovaries, including some salvageable ones, look deceptively worse (blue-black) macroscopically than they appear to be histologically. In this study, we examined the histological appearance of torted ovaries and found a follicular preservation rate of 63% (42/67, OPS-6&10), irrespective of appearance at surgery. The preserved oocyte follicles are located in the ovarian cortex, and embedded within the cyst wall. This finding is in agreement with previous studies that have reported reduced fertility in women who had undergone cystectomy for benign pathology [10,11].
An OPS is based on the objective assessment of histological parameters, including the presence of inflammation and preserved oocytes. TNS represents the percentage necrosis/infarction in ovarian specimens. Even in the worst affected ovaries (OPS-4), there was some preservation.
OPS describes the progress of damage histologically. The structural integrity of the germinal layer including the follicles, aims to predict the potential for future viability. This data has a dual purpose: i) Empirical clinical prognostic tool. For example, when encountering a post-menopausal woman with ovarian torsion, there is a 70% chance that the ovary will be mostly necrotic (OPS-4). ii) Use OPS in pathology reporting of ovarian torsion. Small amounts of tissue from a biopsy or cystectomy is sufficient. Considering that 25/54 (46%) were young nulliparous women, OPS could be investigated as a prognostic tool for future fertility potential. For example, if a young woman had an ovarian detorsion (+/-cystectomy/biopsy) of a blue-black looking ovary, which then shows benign pathology and OPS-10, then this should be re-assuring.
This data provides an evidence-base for individualising the management of patients according to their clinical situation.
The highly significant association between TNS-cortical with OPS, confirmed the temporal relationship of events in the progression of ovarian ischaemia to infarction. It is clear that the ovarian medulla undergoes changes of ischaemia and infarction first, followed by a slow gradual involvement of the ovarian cortex, which contains oocytes for future maturation. This relative resilience of the germinal tissue and follicles in an excised torted cyst (or oophorectomy specimen) could also possibly predict the viability of follicles in the native conserved ovary.
Some clinical predictors of ovarian torsion diagnosis, were also predictors of low OPS. Fever >38°C, leucocytosis>14.9 k/ul and CRP >75 mg/l were associated with worse histological appearances (OPS-4&6). These results support factors considered during clinical decision-making. Pregnant and post-menopausal women are more likely to have lower OPS 3/5 (60%) had OPS-4&6. Prolonged torsions >70 hours had lower preservation (OPS-4&6). Other described diagnostic predictors, including vomiting, leucorrhoea and menorrhagia and cyst size were not found to be predictors of histological preservation [12].
In rodents, peritonitis and sepsis occurred after the 36 hr watershed time [9]. The torsions in the animal model were all identically performed by a described technique, therefore there was no variation in tightness at the neck of the torsion, and no other co-existing pathology and all other variables such as age were controlled. Re-operation for ovarian infarction after detorsion has been reported in a young patient undergoing fertility treatment [13]. Therefore, even-though the ovarian preservation scores are optimistic; caution should be exercised as a third of cases had histological evidence of peritonitis, as evidenced by the presence of fibrinous exudate.
Chen et al., observed a 19% (13/69) incidence of torsion in previously normal ovaries [6]. A comparable figure from our study is 34% (23/67). We found 2.9% were isolated tubal torsions, which compares to a rate of 5.5% found in other series [5]. Dermoid cyst (25%) was the most common underlying pathology in a torted ovary, which is comparable to other studies [14]. All patients except one showed a benign pathology. The exception was a granulosa-cell tumour in a post-menopausal woman. While considering fertility sparing treatment options, it is reassuring to note that malignant pathology is a rare occurrence in torted ovaries, especially in women of reproductive age. In postmenopausal women, the sub-acute or even chronic presentation of benign torted ovarian masses mimicked the radiological appearance of malignancy. This led to more extensive cancer staging surgery for benign pathology.
Ovarian torsion most commonly presents with severe colicky pain in the corresponding iliac fossa or lower abdomen, with 57% requiring opiate analgesia at presentation. However, there is a group of patients whose symptoms are less severe and have a slower onset. These patients present a diagnostic challenge. They often present with mild to moderate pain several times leading to a delayed diagnosis. Interestingly, these patients are over-represented in OPS-10. Half the patients in the OPS-10 group had duration of torsion less than 70 hours (7-70 hours) and half over (120-12000 hours). The patients with an acute short presentation had a high analgesia requirement (WHO-4). It is evident that there are two distinct patient subsets in the OPS-10 group: an acute and a chronic presentation. One patient with OPS-10, was asymptomatic, where a dermoid cysts bilaterally were found incidentally during an abdominal screening ultrasound for liver transaminitis whilst on statins.
Other variables that may impact ovarian preservation include the number of twists, and tightness at the neck of the torsion, and the nature of coexisting ovarian masses. The combination of these factors may contribute to the overall outcome. Currently, there is no clinical tool available to assess the tightness at the neck of the torsion. These factors may have played a role in at least two of our patients; one had prolonged torsion (>12,000 hrs), but revealed a fully viable ovary (OPS-10, TNS<10%); the second patient was asymptomatic described above (OPS-10 bilaterally).
Long ovarian ligaments have been suggested as a possible cause for torsion, however this has not been proven, and there is no clinical tool of measuring the length of the ovarian ligaments at laparoscopy. Oophoropexy has been suggested to prevent recurrence of torsion [15]. However, only a small number of patients are reported in the literature, with variable success [16].
Ovarian torsion is often compared to testicular torsion, despite their major anatomical, embryological, histological and patho-physiological differences [17]. It is worth noting that unlike the testicle, the ovary has an extensive dual blood supply (from the ovarian artery and the anastomosis with the uterine artery via. the broad ligament). Histologically, ovaries and testicles are entirely different, with testicles being a collection of tubules, and the ovary being a stroma of cells. In addition, untreated testicular torsion leads to sympathetic orchidopathia, where the blood-testis immunological barrier is disrupted, leading to anti-sperm auto-antibody formation, leading to bilateral testicular atrophy, and male-factor infertility [18,19]. This phenomenon has not been described in the ovary. Therefore, the differences exceed the similarities.
This series has 77% oophorectomy rate, and reflects current practice in the UK. Even though, some data in support of detorsion has been published for over a decade, it has had only a modest impact upon clinical practice. A more sophisticated approach towards the management of ovarian torsion is needed to provide individualised care in clinical practice. One particular method of management is unlikely to be suitable for all patients, and in all situations. OPS and TNS may be used as clinical tools, as well as a standardised method of evaluating ovarian torsion in research.
High histological preservation demonstrated by OPS should translate to better future fertility potential. High TNS equates to low OPS, and therefore to the overall degree of ovarian tissue damage that may translate to the risk of post-operative re-intervention for severe ovarian infarction. It is beyond the scope of this study to attempt to explore how the histological structural integrity of follicles translates to functional preservation. Clinically, an ultrasound follow-up study has shown follicular formation after detortion [7]. Oocytes were subsequently stimulated and retrieved from previously detorted ovaries for in-vitro fertilisation, leading to successful assisted pregnancies [7].
Conclusion
Ovaries appear black-blue due to vascular haemorrhagic congestion, which is not necessarily associated with necrosis. There was a more than expected overall rate of ovarian histological preservation (62%), irrespective of the appearance at surgery. Pyrexia, and raised inflammatory markers signify more extensive ovarian damage (OPS-4&6). The rarity of malignant pathology in the pre-menopausal cohort may justify a conservative approach in selected cases. Prolonged torsion (>70 hours) in patients presenting acutely, is associated with poor histological preservation. Post-menopausal patients had pre-operative radiological appearances suspicious of malignancy, and showed poor preservation (70%, OPS-4).
References
- Houry D, Abbott JT (2001) Ovarian torsion: a fifteen-year review. Ann Emerg Med 38: 156-159. [Crossref]
- Matthews MAB, Raval MV, Watkins DJ, King D (2014) Diagnosis and management of an ovarian cyst complicated by in utero: A case report. J Pediatr Surg Case Reports 2: 20-22.
- Piper HG, Oltmann SC, Xu L, Adusumilli S, Fischer AC (2012) Ovarian torsion: diagnosis of inclusion mandates earlier intervention. J Pediatr Surg 47: 2071-2076. [Crossref]
- Hibbard LT (1985) Adnexal torsion. Am J Obstet Gynecol 152: 456-461. [Crossref]
- Kupesic S, Plavsic BM (2010) Adnexal torsion: color Doppler and three-dimensional ultrasound. Abdom Imaging 35: 602-606. [Crossref]
- Chen M, Chen CD, Yang YS (2001) Torsion of the previously normal uterine adnexa. Evaluation of the correlation between the pathological changes and the clinical characteristics. Acta Obstet Gynecol Scand 80: 58-61. [Crossref]
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, et al. (2003) Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod 18: 2599-2602. [Crossref]
- Shalev E, Bustan M, Yarom I, Peleg D (1995) Recovery of ovarian function after laparoscopic detorsion. Hum Reprod 10: 2965-2966. [Crossref]
- Taskin O, Birincioglu M, Aydin A, Buhur A, Burak F, et al. (1998) The effects of twisted ischaemic adnexa managed by detorsion on ovarian viability and histology: an ischaemia-reperfusion rodent model. Hum Reprod 13: 2823-2827. [Crossref]
- Li CZ, Liu B, Wen ZQ, Sun Q (2009) The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cysts: a prospective clinical study of 191 patients. Fertil Steril 92: 1428-1435. [Crossref]
- Celik HG, Dogan E, Okyay E, Ulukus C, Saatli B, et al. (2012) Effect of laparoscopic excision of endometriomas on ovarian reserve: serial changes in the serum antimüllerian hormone levels. Fertil Steril 97: 1472-1478. [Crossref]
- Huchon C, Staraci S, Fauconnier A (2010) Adnexal torsion: a predictive score for pre-operative diagnosis. Hum Reprod 25: 2276-2280. [Crossref]
- Pryor RA, Wiczyk HP, O'Shea DL (1995) Adnexal infarction after conservative surgical management of torsion of a hyperstimulated ovary. Fertil Steril 63: 1344-1346. [Crossref]
- Balci O, Icen MS, Mahmoud AS, Capar M, Colakoglu MC (2011) Management and outcomes of adnexal torsion: a 5-year experience. Arch Gynecol Obstet 284: 643-646. [Crossref]
- Fuchs N, Smorgick N, Tovbin Y, Ben Ami I, Maymon R, et al. (2010) Oophoropexy to prevent adnexal torsion: how, when, and for whom? J Minim Invasive Gynecol 17: 205-208. [Crossref]
- Sheizaf B, Ohana E, Weintraub AY (2013) "Habitual adnexal torsions"--recurrence after two oophoropexies in a prepubertal girl: a case report and review of the literature. J Pediatr Adolesc Gynecol 26: e81-84. [Crossref]
- Asfour V (2013) Time urgency in ovarian versus testicular torsion. TOG 15:138.
- Wyburn-Mason R (1981) Sympathetic orchiopathia. Lancet 2: 1417-1418. [Crossref]
- Isidori A, Dondero F, Lenzi A (1988) Immunobiology of male infertility. Hum Reprod 3: 75-77. [Crossref]


