Abstract
Diabetes mellitus is a group of metabolic diseases characterized by high blood glucose level that result from defects in insulin secretion, action, or both. Hyperglycemia was induced oxidative stress and become the main factor for predisposing the complication in diabetes. This study was designed to investigate the effect of garlic on alloxan induced insulin secretion, metabolic disorder and oxidative stress in diabetic rats. The present study includes blood glucose, body weight, total cholesterol, triglyceride, uric acid, creatinine, insulin, c-peptide level and tissue histology in all groups. Diabetic rats increase the level of blood glucose, total cholesterol, triglyceride, uric acid, creatinine and decrease the level of insulin, c-peptide body weight and tissue architecture as compared to control group. Diabetic rats treated with aqueous suspension of garlic improve the secretion of insulin, repaired metabolic disorder, reduce oxidative stress and prevented all these observed abnormalities. Aqueous suspension of garlic showed antidiabetic properties.
Key words
diabetes, β cell, alloxan, Allium sativum, oxidative stress
Introduction
Diabetes is a disease associated with glucose metabolism resulting from defect in insulin secretion, action and both [1]. The complications of diabetes are linked to oxidative stress induced by hyperglycemia and it is decrease the body natural antioxidant system [2,3]. Alloxan, a chemical used to induce experimental diabetes mellitus, causes the beta cells of the islets of langerhans to swell and finally degenerate. Alloxan diabetic rats have been reported to have increased vascular permeability, nerve fibber loss. The mechanism of action of this chemical is via free radical induced tissue damage, causing oxidative stress in the tissue involved, specifically the pancreas.
Alloxan is a toxic glucose analogue, which selectively destroys insulin-producing cells in the pancreas when administered to rodents and many other animal species. This causes an insulin-dependent diabetes mellitus in these animals, with characteristics similar to type 1 diabetes. Alloxan is selectively toxic to insulin-producing pancreatic beta cells because it preferentially accumulates in beta cells through uptake via the GLUT2 glucose transporter. Alloxan, in the presence of intracellular thiols, generates reactive oxygen species (ROS) in a cyclic reaction with its reduction product, dialuric acid. The beta cell toxic action of alloxan is initiated by free radicals formed in this redox reaction [4]. Alloxan induced free radical damage tissue, causing oxidative stress in the tissue involved, specifically the pancreas. Increase the level of oxidative stress induced damage in cellular macromolecule such as lipid, carbohydrate and protein. Alloxan is known to produce oxidative stress by changing the activity of antioxidant enzymes and increasing the production of ROS.
The pancreas releases insulin under high glucose tension to increase its uptake and utilization by cells. Damaged islets cells of the pancreas, especially when the β cells are affected, could result in impairment of insulin release and as such affect blood glucose level. Rats in the experimental group showed a significantly high blood glucose level following alloxan induction revealing the toxicity of the chemicals. Treated group, showed the protective effect of the extract on diabetic rats, plant extract has been characterized to contain antioxidants including, flavonoids, phenolics, alkaloids and some glycosides [5]. There is a positive correlation between flavonoids component of a plant extract and its ability to restore free radicals damaged cells.
Garlic (Allium sativum) is one of the most popular herbs used worldwide to reduce various risk factors associated with several diseases [6]. Actually, garlic contains a variety of effective compounds that exhibit anticoagulant (anti-thrombotic) [7,8], antioxidant [9,10], antibiotic [11], hypocholesterolaemic [12] and hypoglycemic as well as hypotensive activities [13,14]. Most of the studies showed that garlic can reduce blood glucose levels in diabetic rats [15]. Augusti and Sheela consistently showed that S-allyl cysteine sulphoxide, (allicin), a sulphurcontaining amino acid in garlic had a potential to reduce the diabetic condition in rats almost to the same extent as glibenclamide and insulin [16,17].
The present study was aimed to evaluate the possible cytoprotective effect of aqueous suspension of Allium sativum and determine the antidiabetic propertes of Allium sativum in type I diabetic rats, compared to normal control and untreated diabetic rats.
Materials and methods
Experimental animal
Male albino rats of Wistar strain (weight 120 ± 20g) was used in the proposed study. Animals were obtained from the animal facilities of Defence Research and Development Establishment, Gwalior, India, and was maintained under controlled conditions of temperature (25 ± 2°C), relative humidity of (50 ± 15%), and normal photoperiod (light-dark cycle of 12 hrs) in the animal room of our department on standard pellet diet and tap water ad libitum. Animals was housed throughout the experiment in polypropylene cages containing paddy husk as bedding and allowed to acclimatize to the environment of animal room for 7 days before the start of experiment. Aimals were handled, ethically treated and humanly killed as per the rules and instructions of Ethical Committee of Animal Care of Jiwaji University, Gwalior, India, in accordance with the Indian National law on animal care and use.
Experimental design
Twenty four rats were randomly divided into four groups of six rats each. Animals were divided into four groups and were given following treatments: Group 1: Control (normal blood glucose level). Group 2: Treated control group (treated with Allium sativum extracts 0.25 mg/kg body weight). Group 3: Diabetic (I.V. injection of alloxan 70 mg/kg body weight). Group 4: Treated diabetic group (treated with Allium sativum extracts 0.25 mg/kg body weight).
Induction of experimental diabetes and plant extract treatment
Type 1 diabetes was induced by giving single intravenous injection of alloxan monohydrate 70 mg/kg body weight, dissolved in 0.9% solution of sodium chloride [18]. The animals were checked for blood glucose level 48 h after alloxan injection, and blood sugar level above 200 mg/dl was used for the experiment.
Allium sativum (Garlic) seeds were purchased from the local herbal market, cleaned, and aqueous suspension of Allium sativum seeds was prepared freshly for each day and 0.25 mg/kg body weight was given treated rats for 1 Hrs at 37̊°C and two weeks treatment for histological analysis.
Blood sample collection
Blood was collected at after 14 days from eye orbital of rats with the help of capillary glass tube and centrifuged 1000 rpm for 10 min. at 4°C and serum sample collected. Serum sample all groups were analysed for various biochemical parameters at same time after 14 days of feeding.
Biochemical parameters
Fasting blood glucose levels were estimated by glucose oxidase peroxidase reactive strips [19] (Accu-Chek, Roche Diagnostics, USA). The biochemical parameters evaluated were serum lipid profiles [20,21] (Triglyceride and Total cholesterol), kidney biomarkers such as creatinine [22] using diagnostics kits. The c-peptide and insulin level was estimated by ELISA using Rat Insulin Kit.
Estimation of glucose, total cholesterol, triglyceride Uric acid and creatinine
The lipid profile parameters such as total cholesterol (Cholesterol oxidase- peroxidase method) and serum triglyceride (GPO-POD Method) were calculated from Freidewald’s Formula all the estimation were carried out fasting serum samples using commercial kits manufactured by Crest Biosystems India Pvt. Ltd.
The kidney function test such as serum Uric acid, creatinine (Mod. Jaffe’s Kinetic method) were estimated by using kits manufactured by Crest Biosystems, India, Pvt. Ltd.
Estimation of insulin and C-peptide
Quantitative estimation of serum insulin was done by rat insulin ELISA kits. The sensitivity of the kit is 0.025 μg/l. It is a solid phase two-site enzyme Immunoassay. It is based on the direct sandwich technique in which two monoclonal antibodies are directed against separate antigenic determinants on the insulin molecule. During incubation, insulin in the sample reacts with peroxidase-conjugated anti-insulin antibodies and anti-insulin antibodies bound to microtitration well. A simple washing step removes unbound enzyme loaded antibody. The bound conjugate was detected by reaction with 3, 3’, 5, 5’-tetramethylbenzidine. The reaction was stopped by adding acid and read using a Elisa reader.
Histopathology
For histopathological analyses, tissues were collected at the time of sacrifice, freed from fat bodies, washed with normal saline and fixed in Bouin’s fluid for 12–24 h. After fixation, tissues were washed overnight under running tap water to remove excess fixative, and embedded in paraffin blocks. Paraffin blocks were cut at 4 µm for liver and pancreas and at 12 µm for brain with the help of semi-automated microtome (Leica EG 1106 Microtome). Six slides per tissue were prepared and stained with hematoxylin and eosin [23]. Stained tissue sections were mounted in DPX, covered with cover slip and viewed under light microscope at 10x magnification (Leica Optiphase microscope).
Results and discussion
The induction of experimental diabetes in the rats using chemicals which selectively destroy pancreatic β cells is very convenient and simple to use. Type 1 diabetes mellitus (T1DM) is an autoimmune disease caused by absolute insulin deficiency due to destruction of the pancreatic β cells. Type I is characterized by progressive β cell failure and recent treatments are focusing on enhancing endogenous β cell function and regeneration. Because apoptosis is probably the main form of β cell death, apoptosis-caused β cell mass decreasing and function impairing has provided a hopeful target in diabetes treatment. It is suggested that cytokines, lipotoxity and glucotoxity are three main stimuli for β cell apoptosis [24].
Herbal extract of various plants examined were probably interfere with either food intake or gastrointestinal glucose absorption [25]. The herbal treatment could also lead to decrease the tendency for the formation of carcinogenesis. Various drugs show a dose dependent reduction in the blood glucose level and also compared well with the effect of insulin [26]. Blood glucose level, cholesterol, triglycerides and blood pressure reduction had also been noted well when oyster mushrooms administrated in the diet of diabetic patient [27]. Various extract of medicinal plants shows antihyperlipidemic and antiperoxidative effect in patients.
Isolation of islets [12,13] has promoted studies related to understanding the pathophysiology of type I diabetes, transplantation, screening of hypoglycaemic drugs and probing into diabetes causing mechanisms, to device effective means of prevention.
The blood glucose level of all the rats was tested by taking the blood from the tail vein and using electronic glucometer. The anti-diabetic effects of the extracts on the fasting blood sugar levels of diabetes (Table 1) and administration of alloxan (70 mg/kg, i.v) led to 4 fold elevation of fasting blood glucose levels, which was maintained for period of 14 days. It was observed that oral administration of Allium sativum extracts significantly decreased the blood glucose levels in diabetic rats. The present study results showed that oral administration of Allium sativum extracts daily for 14 days, to the diabetic rats caused 22% decrease on 7th day and 41.2% decrease in the blood glucose level on 14 days of the start of treatmet (Table 1) and Allium sativum extracts daily for 14 days, to the diabetic rats caused caused 29.2% increase on 7th day and 36.8% increase in the body weight on 14 days of the start of treatmet (Table 2). The results clearly showed the hypoglycemic potential of Allium sativum extracts. Diabetic rats treated with oral administration of Allium sativum extracts for 14 days showed that significantly increased the level of insulin and c-peptide 59% and 43% in diabetic rats as compared with diabetic control rat (Table 3).
Groups |
0 Day |
7th Day |
14th Day |
Control |
103.67 ± 2.4 |
109.00 ± 2.65 |
111.67 ± 3.53 |
Control + Allium sativum |
110.33 ± 2.6# |
107.67 ± 1.76# |
102.67 ± 2.96# |
Diabetic |
413.00 ± 8.14*** |
419.00 ± 6.66*** |
422.33 ± 6.36*** |
Diabetic + Allium sativum |
418.67 ± 4.63*** |
326.67 ± 3.28** |
248.33 ± 2.91*** |
Table 1. Effect of oral treatment of Allium sativum extracts on glucose concentration in alloxan induced diabetic rats.
Few drop of blood was taken from the tail vein and glucose level was measured using electronic glucose meter. Blood glucose levels are expressed as mg/ dl. Results are mean ± S.E. of four set of observation. #p>0.0, *p<0.05, **p<0.01, ***p<0.001 when compared with respective control, comparison between diabetic and diabetic + treatment group; Control and diabetic rats were given aqueous suspension of Allium sativum extracts orally, 0.25 mg/kg body weight with the help of cannula daily for 14 days.
Groups |
0 Day |
7th Day |
14th Day |
Control |
120.33 ± 1.67 |
129.00 ± 1.53 |
135.67 ± 2.34** |
Control + Allium sativum |
127.00 ± 1.15* |
136.33 ± 1.67** |
142.67 ± 0.88*** |
Diabetic |
107.67 ± 1.2** |
90.33 ± 0.88*** |
87.00 ± 1.15*** |
Diabetic + Allium sativum |
111.33 ± 0.88# |
116.67 ± 1.2*** |
119.00 ± 1.15*** |
Table 2. Effect of Allium sativum extracts on the body weight in normal and diabetic rats.
Weight measure with help of weigh balance and expressed in gm. Results are mean ± S.E. of four set of observation. #p>0.05, *p<0.05, **p<0.01, ***p<0.001 when compared with respective control, comparison between diabetic and diabetic + treatment group; Control and diabetic rats were given aqueous suspension of Allium sativum extracts orally, 0.25
mg/kg body weight with the help of cannula daily for 14 days.
Groups |
Insulin in ng/ml |
C-peptide in pmol/l |
Control |
1.97 ± 0.07 |
251.47 |
Control + Allium sativum |
2.19 ± 0.08# |
261.1# |
Diabetic |
0.88 ± 0.06*** |
151.23*** |
Diabetic + Allium sativum |
1.40 ± 0.03** |
216.2*** |
Tobutamide |
2.33 ± 0.09* |
231.83*** |
Table 3. Effect of Allium sativum extracts for 14 days in experimental rats on the levels of insulin and C-peptide in normal and diabetic rats.
Insulin concentration is expressed as ng/ml and C-peptide concentration is expressed as pmol/L. Results are mean ± S.E. of five set of observation. *P<0.05, **P<0.001, ***P<0.0001 and #P>0.05 when compared with respective control, comparison between diabetic and diabetic + treatment group; Control and diabetic rats were given aqueous suspension of Allium sativum extracts orally, 0.25 mg/kg body weight with the help of cannula daily for 14 days.
The results showed total Cholesterol, triglyceride Uric acid and creatinine levels were increased in alloxan induced diabetic rats. Total cholesterol, triglyceride, uric acid and creatinine levels were increase 78%, 106.5%, 104.8% and 126.5% in alloxan induced diabetic rats when compared with control rats (Table 4). Diabetic rats treated with oral administration of Allium sativum extracts for 14 days, caused 35.6%, 39.8%, 46.5% and 35.6% decrease total cholesterol, triglyceride, uric acid and creatinine levels as compared with diabetic rats.
Experiment |
Control |
Control + Allium sativum |
Diabetic |
Diabetic + Allium sativum |
Total cholestrol |
141 ± 2.65 |
136 ± 2.52# |
251.67 ± 3.18*** |
162 ± 2.65*** |
Triglyceride |
93 ± 2.31 |
87.67 ± 3.18# |
192 ± 3.21*** |
115.67 ± 2.6*** |
Uric acid |
1.26 ± 0.05 |
1.17 ± 0.04# |
2.58 ± 0.04*** |
1.38 ± 0.03*** |
Creatinine |
0.98 ± 0.04 |
0.90 ± 0.03# |
2.22 ± 0.05*** |
1.43 ± 0.03*** |
Table 4. Effect of Allium sativum extracts for 14 days in experimental rats on the levels of Total Cholesterol, Triglyceride, uric acid and Creatinine in normal and diabetic rats.
Total cholesterol, Triglyceride, Uric acid and creatinine concentration is expressed as mg/dl. Results are mean ± S.E. of four set of observation. *P<0.05, **P<0.001, ***P<0.0001 and #P>0.05 when compared with respective control, comparison between diabetic and diabetic + treatment group; Control and diabetic rats were given aqueous suspension of Allium sativum extracts orally, 0.25 mg/kg body weight with the help of cannula daily for 14 days.
Result from the pancreatic control and control treatments (Allium sativum) tissue rats showed normal acini, architecture of pancreas (Figures 1a & 1b) and no histopathological alterations were observed in these animals. Pancreatic section from diabetic rats (Figure 1c) showed extensive damage to the islets of langerhans and reduced dimensions of islets. When the diabetic rats were given Allium sativum oral treatment for two weeks and its is show regenerating tiny islets (Figure d) which could be comparable to that of non-diabetic control rats. These histological observations showed the protective role of polyphenolic extract on pancreas in alloxan induced diabetic rats (Figure 1).
2021 Copyright OAT. All rights reserv
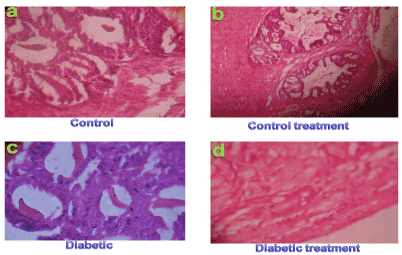
Figure 1.Histological studies of pancreatic tissues of control (a), control treatment (b), alloxan induced diabetic (c) and Allium sativamextracttreated diabetic (d) rats
Conclusion
Alloxan induced diabetes has negative effects on the activities of antioxidant enzymes and inhibited insulin secretion and oxidative stress in diabetic rats. Oral administration of raw garlic extracts insulin secretion and metabolic complications along with oxidative stress in diabetic rats. The results clearly showed the hypoglycemic potential of garlic extracts. Further studies are necessary to found the active component of garlic, role of these herbal drugs in controlling type I diabetes and its complications.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Mr. SKJ has received stipend from the Department of Higher Education, Government of M.P., Bhopal, India. The financial support from the Department of Science and Technology, N. Delhi, India, in the form of FIST grant to the school is thankfully acknowledged.
References
- WHO (1999) Definition Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes. WHO/NCD/NCS Geneva: 1-58.
- Kikkawa R, Koya D, Haneda M (2003) Progression of diabetic nephropathy. Am J KidneyDis 41: S19-21. [Crossref]
- Udoh AE, Ntu I, Essien O, Ndon M (2007) Red cell catalase activity in diabetics. Pak J Nutr 6: 511-515.
- Lenzen S (2008) The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia51: 216-226. [Crossref]
- Youdim K, McDonald J, Kalt W, Joseph JA (2007) Potential role of dietary flavonoids in reducing micro-vascular endothelium volubility to oxidative and inflammatory results. JNutrBiochem13: 282-288. [Crossref]
- Thomson M, Al-Amin ZM, Al-Qattan KK, Shaban LH, Ali M (2007) Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metabolism; 15: 108-115.
- Augusti KT, Sheela CG (1996) Antiperoxide effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in diabetic rats. Experientia52: 115-120. [Crossref]
- Anwar MM, Meki AR (2003) Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp BiochemPhysiol A MolIntegrPhysiol135: 539-547. [Crossref]
- Bakri IM, Douglas CW (2005) Inhibitory effect of garlic extract on oral bacteria. Arch Oral Biol50: 645-651. [Crossref]
- Rees LP, Minney SF, Plummer NT, Slater JH, Skyrme DA (1993) A quantitative assessment of the antimicrobial activity of garlic (Allium sativum). World J MicrobiolBiotechnol9: 303-307. [Crossref]
- Kiesewetter H, Jung F, Pindur G, Jung EM, Mrowietz C, et al. (1991) Effect of garlic on thrombocyte aggregation, microcirculation, and other risk factors. Int J ClinPharmacolTherToxicol29: 151-155. [Crossref]
- Ali M, Thomson M (1995) Consumption of a garlic clove a day could be beneficial in preventing thrombosis. Prostaglandins LeukotEssent Fatty Acids 53: 211-212. [Crossref]
- Ali M, Al-Qattan KK, Al-Enezi F, Khanafer RM, Mustafa T (2000) Effect of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet. Prostaglandins LeukotEssent Fatty Acids 62: 253-259. [Crossref]
- Banerjee SK, Maulik SK (2002) Effect of garlic on cardiovascular disorders: a review. Nutr J 1: 4. [Crossref]
- Bordia T, Mohammed N, Thomson M, Ali M (1996) An evaluation of garlic and onion as antithrombotic agents. Prostaglandins LeukotEssent Fatty Acids 54: 183-186. [Crossref]
- Augusti KT (1996) Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.). Indian J ExpBiol34: 634-640. [Crossref]
- Block E, Ahmad S, Catalfamo JL, Jain MK, Apitz-Castro R (1986) Antithrombotic organosulfur compounds from garlic, structural, mechanistic and synthetic studies. J Am ChemSoc 108: 7045-7055.
- Rahimi R, Nikfar S, Larijani B, Abdollahi M (2005) A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother59: 365-373. [Crossref]
- Veiga F, Fernandes C, Teixeira F (2000) Oral bioavailability and hypoglycaemic activity of tolbutamide/cyclodextrin inclusion complexes. Int J Pharm202: 165-171. [Crossref]
- Van Handel E, ZilVersmit DB (1957) Micromethod for the direct determination of serum triglycerides. J Lab Clin Med50: 152-157. [Crossref]
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. ClinChem20: 470-475. [Crossref]
- Bowers LD (1980) Kinetic serum creatinine assays I. The role of various factors in determining specificity. ClinChem26: 551-554. [Crossref]
- McManus JFA, Mowry RW (1960) Staining methods: histologic and histochemical.Paul B. Hoeber, USA.
- Cerasi E, Kaiser N, Leibowitz G (2000) [Type 2 diabetes and beta cell apoptosis]. Diabetes Metab 26 Suppl 3: 13-16. [Crossref]
- Musabayane CT, Cooper RG, Rao PV, Balment RJ (2000) Effects of ethanol on the changes in renal fluid and electrolyte handling and kidney morphology induced by long-term chloroquine administration to rats. Source Alcohol 22:129-138. [Crossref]
- Gundidza M, Masuku S, Humphrey G, Magwa ML (2005) Anti-diabetic activity of Aloe excelsa. Cent Afr J Med 51: 115-120. [Crossref]
- Khatun K, Mahtab H, Khanam PA, Sayeed MA, Khan KA (2007) Oyster mushroom reduced blood glucose and cholesterol in diabetic subjects. Mymensingh Med J16: 94-99. [Crossref]

