Abstract
Background: Black ginger (Kaempferia parviflora) contains polymethoxyflavones, which are flavonoids that exhibit various bioactivities including improvements in muscular metabolism. We examined the effects of black ginger extract (KPE) rich in polymethoxyflavones on physical fitness and fatigue. Methods: 24 healthy volunteers were recruited. They were randomly divided into two groups: group A received KPE (30 mg/day) and then a placebo while group B received a placebo and then KPE in a crossover trial. Each volunteer took one capsule containing KPE or the placebo once a day for 4 weeks. A physical fitness test (PFT), questionnaire, and blood test were performed at 0, 4, 7, and 11 weeks (wash-out term for 3 weeks). Results: After a 4-week ingestion period, improvements in left hand grip strength (2.80 vs 0.03 kg), performance in the 30-second chair stand test (6.27 vs 1.71 times), 5-m tandem walking test (-3.17 vs -0.87 sec), and cycle ergometer test (8.54 vs 1.13 kcal) were significantly greater in the KPE group than in the placebo group. In a fatigue of subjects without an exercise habit, the mean reductions after ingestion of KPE in the daily VAS fatigue score (-9.87 vs +1.52%), post PFT VAS fatigue score (-10.2 vs -0.91%), and chronic fatigue syndrome (CFS) score (-3.93 vs -2.47) were greater than the placebo group. Conclusions: The ingestion of KPE was found to enhanced physical fitness, namely, grip strength, leg strength, balance, endurance, and locomotor activity. Furthermore, KPE intake slightly improved fatigue at the conventional state and post PFT state as well as CFS scores in subjects without an exercise habit.
Key words
randomized, double-blind, placebo-controlled crossover trial, kaempferia parviflora, polymethoxyflavone,exercise,physical fitness, fatigue
List of abbreviations
AICAR: 5-aminoimidazole-4-carboxyamide ribonucleotide; AMPK : AMP-activated protein kinase; KPE: black ginger (Kaempferia parviflora) extract; BCAA: branched chain amino acids; CFS: chronic fatigue syndrome; MCV: mean cell volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; PFT: physical fitness test; PMFs: polymethoxyflavones; QOL: quality of life; TG: triglyceride; VAS: visual analog scale
Backgrounds
Locomotive dysfunctions, which are related to muscles, bones, and joints, account for some of the main factors impairing the quality of life (QOL) of individuals. Muscular dysfunctions including sarcopenia syndrome may trigger locomotive dysfunctions and are caused by declines in muscular mass and metabolism, which are induced by aging or insufficient daily exercise [1-5]. Therefore, continual exercise is necessary in order to prevent locomotive dysfunctions [6-9]. In addition, supportive strategies to enhance exercise performance such as ingestion of nutrients may be effective. Amino acids and peptide derivatives such as branched chain amino acids (BCAA) and imidazole peptides are often used in dietary supplements prescribed to improve muscle function. These nutritional ingredients were previously reported to be effective in clinical trials [10]. One of the mechanisms responsible is an increase in the mass of skeletal muscle through protein synthesis. On the other hand, co-enzyme Q10 [11-14], L-carnitine [15,16], and polyphenol [17] have been reported as ingredients that effectively improve muscular metabolism and reduce oxidation stress.
Black ginger, the rhizome of Kaempferia parviflora (Zingiberaceae) has traditionally been used in folk medicines and nourishing foods in Thailand, and contains polymethoxyflavones (PMFs), which are flavonoids that exhibit various bioactivities (i.e. anti-inflammatory, antioxidant [18-21], anticancer [22,23], muscular metabolism-enhancing [24], anti-photoaging [25], (PDE)5 inhibitory [26], anti-cardiovascular disease [27], and viral protease inhibitory [28] activities). A large number of studies have investigated the bioactivities of black ginger extract (KPE), which is rich in PMFs [24,29-37]. We also reported that PMFs in KPE improved muscular metabolism through AMP-activated protein kinase (AMPK) activation in myocytes [24]. Furthermore, clinical trials have been conducted on the effects of KPE physical performance in athletes and elderly subjects [36,37].
However, clinical reports have yet to be performed on healthy adults aged between 20 and 65 years old. In addition, the effects of KPE on subjects without an exercise habit or on post-exercise fatigue have not yet been investigated. Therefore, we examined the effects of KPE on physical fitness and fatigue in healthy volunteers.
Methods
Test design
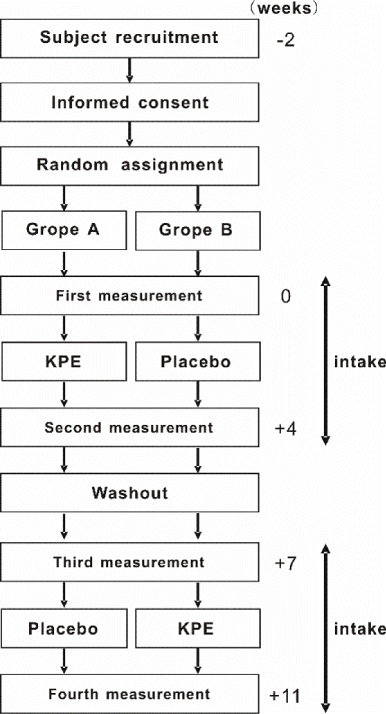
This study was performed as a randomized double-blind placebo controlled crossover trial guided by the directions of the 6th revision of the Declaration of Helsinki (2008) and guidelines of the consolidated standards of reporting (CONSORT 2010 Statement, Japan). In addition, the study was registered in the University Hospital Medical Information Network (UMIN, ID: UMIN000021051). The protocol was approved by the Ethics Committee in Oryza Oil & Fat Chemical Co., Ltd. (October 1, 2015, Approval No.20151001) without contribution to the trial. The study was performed based on the schedule described in Figure 1.
Subjects
Subjects were recruited as healthy volunteers from Oryza Oil & Fat Chemical Co., Ltd., and were aged between 20 and 65 years old. Key exclusion criteria were 1) subjects receiving medication or having anamnesis of serious diseases requiring medication, 2) subjects with chronic diseases including asthma and 3) subjects who were allergic to the test sample.
The essential number of subjects was determined as 23 by G*Power, software for a power analysis. Twenty four subjects (male: 16, female: 8) received full explanations on the purpose and method of the study, and then participated in the trial with their consent. Male and female subjects were randomly distributed into groups A and B in order of age by a third member. Information on the assignment was concealed by the third member until fixation of the data. Subject backgrounds in each group were shown in Table 1.
Table 1. Subject backgrounds
General characteristics |
Group A |
Group B |
Total |
All subjects |
|
|
|
Male |
8 |
8 |
16 |
Female |
4 |
4 |
8 |
Total |
12 |
12 |
24 |
Ages (year) |
35.4 ± 3.5 |
35.0 ± 2.9 |
35.2 ± 2.2 |
Subjects with an exercise habit |
|
|
|
Male |
5 |
3 |
8 |
Female |
0 |
1 |
1 |
Total |
5 |
4 |
9 |
Ages (year) |
33.6 ± 3.2 |
37.8 ± 3.3 |
35.4 ± 2.2 |
Subjects without an exercise habit |
|
|
|
Male |
3 |
5 |
8 |
Female |
4 |
3 |
7 |
Total |
7 |
8 |
15 |
Ages (year) |
35.4 ± 3.5 |
35.0 ± 2.9 |
35.1 ± 2.3 |
First sample |
KPE |
Placebo |
|
Second sample |
Placebo |
KPE |
|
Data for ages were presented as the mean ± S.E.
Test samples
KPE was prepared according to our previously reported method [24]. KPE was obtained from the dried rhizomes of black ginger by extracting with aqueous ethanol (yield 15.9%). KPE was mixed with modified starch at a ratio of 3:7 (KPE: modified starch) and then powdered by spray drying. Powdered KPE (100 mg) was packed into a capsule. The contents of total PMFs in the KPE capsule were determined by reverse-phase HPLC using a Prominence HPLC system (Shimadzu, Kyoto, Japan) equipped with a photodiode array detector (Model SPD-M20A) and Develosil RPAQUEOUS-AR-5 column (4.6 × 150 mm, 5-mm particle size, Nomura Chemical Co., Ltd., Japan). The mobile phase was a binary gradient and consisted of a mixture of acetonitrile, water, and acetic acid (35: 62.5: 2.5, v/v) as solvent A and a mixture of acetonitrile and acetic acid (97.5: 2.5, v/v) as solvent B. The flow rate was fixed at 1.0 mL/min and the column temperature was set at 35 °C. Gradient conditions were as follows: 0–20 min (solvent A: 99–1%). UV detection at 263 nm was used. The contents of the determined PMFs were 5-hydroxy-3,7-dimethoxyflavone (0.66%), 5-hydroxy-7-methoxyflavone (0.52%), 5-hydroxy-3,7,4'-trimethoxyflavone (0.81%), 5-hydroxy-3,7,3',4'-tetramethoxyflavone (0.30%), 3,5,7,3',4'-pentamethoxyflavone (2.94%), 5,7,4'-trimethoxyflavone (3.14%), 3,5,7,4'-tetramethoxyflavone (1.75%), and 5,7-dimethoxyflavone (2.50%), respectively. In the placebo, modified starch (100 mg) was packed into a capsule. Subjects took one KPE or placebo capsule once a day for 4 weeks.
Physical fitness test (PFT)
The hand grip strength test and 30-second chair stand test were performed according to the methods described in a previous study [37]. In the 5-m tandem walking test, the time to walk 5 m with tandem steps was measured. In the cycle ergometer test, energy consumption (kcal) was measured using an exercise bike (aerobike EZ101, Konami Sports Club Co. Ltd., Tokyo, Japan). Each subject determined the pedal load by themselves, peddled at more than 60 rpm for 10 min, and then peddled with full power for 10 seconds every 2 minutes (a total of 5 times). Locomotor activity (energy consumption: kcal) depended on the load and the peddling times for 10 min.
Questionnaire
Fatigue was evaluated by a visual analog scale (VAS) fatigue score and chronic fatigue syndrome (CFS) score. The evaluation of VAS was performed in the conventional state and post PFT state. The CFS score [38] was determined using the questionnaire in Table 2.
Table 2. Questionnaire for CFS
Questionnaire |
1) |
Do you have problems with tiredness? |
2) |
Do you need to rest more? |
3) |
Do you feel sleepy or drowsy? |
4) |
Do you have problems starting things? |
5) |
Do you start things without difficulty, but get weak as you continue? |
6) |
Are you lacking in energy? |
7) |
Do you have less strength in your muscles? |
8) |
Do you feel weak? |
9) |
Do you have difficulty concentrating? |
10) |
Do you have problems thinking clearly? |
11) |
Do you make slips of the tongue when speaking? |
12) |
Do you find it more difficult to find the correct word? |
13) |
How is your memory? |
14) |
Have you lost interest in the things you used to do? |
Answer |
|
1) |
Better than usual (0 point) |
2) |
No more than usual (1 point) |
3) |
Worse than usual (2 points) |
4) |
Much worse than usual (3 points) |
This questionnaire was previously described by Chalder et al. [38]. Subjects scored “better than usual” (0 point), “no more than usual” (1 point), “worse than usual” (2 points), and “much worse than usual” (3 points) for each question. The total number of points was defined as the CFS score.
Blood test
After PFT and the questionnaire, blood was collected from subjects, and the following parameters were analyzed: total bilirubin, total protein, albumin, A/G ratio, AST, ALT, ALP, LDH, gGTP, CPK, HDL-cholesterol, LDL-cholesterol, total cholesterol, triglyceride (TG), phospholipids, free fatty acids, Na, Cl, K, urea nitrogen, creatinine, uric acid, glucose, ketone bodies, HbA1c, and cortisol. In addition, the number of leukocytes and red blood cells, hemoglobin level, hematocrit, mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count were determined.
Data analysis
Data are presented as the mean ± S.E. In statistical comparisons of PFT with the placebo, questionnaire, and blood test, a paired t-test or Mann-Whitney's U test was performed.
Results
Physical fitness and endurance (all subjects)
In order to evaluate the effects of KPE on physical fitness and endurance, PFT consisting of a hand grip strength test, 30-second chair stand test, 5-m tandem walking test, and cycle ergometer test, was performed. In Table 3, grip strength after a 4-week treatment with KPE significantly increased (right: +2.2 kg, P < 0.05, left: +2.8 kg, P < 0.01) from baseline. Significant improvements were also observed in the 30-second chair stand test (+6.3 times, P < 0.01), 5-m tandem walking test (-3.2 sec, P < 0.05), and cycle ergometer test (+8.6 kcal, P < 0.01). The grip strength of right hand was significantly higher after the ingestion of KPE (44.6 kg, P < 0.05) than after that of the placebo (43.0 kg).
Table 3. Effects of KPE on physical fitness and fatigue (part 1)
Measured parameters |
Placebo |
KPE |
Before |
After |
Net change (D) |
Before |
After |
Net change (D) |
All subjects |
|
|
|
|
|
|
Tiredness without exercise (%) |
34.6 ± 4.3 |
32.0 ± 3.5 |
− 2.57 ± 4.5 |
34.5 ± 4.0 |
29.4 ± 4.3 |
− 5.14 ± 4.2 |
Grip strength (R) (kg) |
42.8 ± 2.5 |
43.0 ± 2.5 |
0.21 ± 0.7 |
42.4 ± 2.4 |
44.6 ± 2.6 *,† |
2.17 ± 0.9 |
Grip strength (L) (kg) |
40.1 ± 2.5 |
40.1 ± 2.5 |
0.03 ± 0.8 |
38.9 ± 2.2 |
41.7 ± 2.2 †† |
2.80 ± 0.8 * |
30-second chair stand test (sec) |
25.3 ± 2.1 |
27.0 ± 1.8 |
1.71 ± 0.9 |
21.4 ± 1.7 |
27.6 ± 1.7 †† |
6.27 ± 1.7 * |
5-m tandem walking test (sec) |
12.2 ± 0.8 |
11.4 ± 0.9 |
− 0.87 ± 0.5 |
13.8 ± 1.3 |
10.6 ± 0.9 † |
− 3.17 ± 1.3 ** |
Cycle ergometer test (kcal) |
47.4 ± 4.5 |
48.6 ± 4.3 |
1.13 ± 1.4 |
44.3 ± 4.0 |
52.9 ± 4.7 †† |
8.54 ± 1.9 ** |
Tiredness after this test (%) |
56.7 ± 4.5 |
51.0 ± 4.9 |
− 5.08 ± 4.3 |
52.7 ± 5.4 |
46.8 ± 3.9 |
− 5.87 ± 4.3 |
Chronic fatigue syndrome score |
16.1 ± 1.2 |
13.7 ± 1.1 |
− 2.38 ± 1.1 |
15.7 ± 1.3 |
13.0 ± 1.2 |
− 2.75 ± 1.2 |
Data were presented as the mean ± S.E (n = 24)
Significant differences from the placebo were indicated as *: P<0.05, **: P<0.01, and those from the baseline were indicated as †: P<0.05, ††: P<0.01 (paired t-test).
Table 4. Effects of KPE on physical fitness and fatigue (part 2)
Measured parameters |
Placebo |
KPE |
Before |
After |
Net change (D) |
Before |
After |
Net change (D) |
Subjects with an exercise habit |
|
|
|
|
|
|
Tiredness without exercise (%) |
34.3 ± 4.0 |
25.0 ± 2.1 |
− 9.38 ± 3.8 |
30.1 ± 2.6 |
32.8 ± 3.9 |
− 2.74 ± 2.6 |
Grip strength (R) (kg) |
50.0 ± 2.1 |
50.1 ± 1.6 |
0.40 ± 0.8 |
50.0 ± 1.9 |
52.2 ± 1.9 † |
2.21 ± 0.5 |
Grip strength (L) (kg) |
47.8 ± 1.9 |
47.2 ± 1.6 |
− 0.60 ± 1.1 |
46.2 ± 1.6 |
48.6 ± 1.6 |
2.39 ± 0.8 |
30-second chair stand test (sec) |
29.9 ± 2.8 |
31.7 ± 1.9 |
1.78 ± 1.3 |
24.2 ± 1.7 |
31.1 ± 1.4 † |
6.89 ± 1.7 |
5-m tandem walking test (sec) |
10.6 ± 0.6 |
9.31 ± 0.4 |
− 1.32 ± 0.4 |
10.2 ± 0.4 |
8.47 ± 0.3 † |
− 1.68 ± 0.3 |
Cycle ergometer test (kcal) |
62.9 ± 4.4 |
65.2 ± 4.0 |
2.27 ± 1.3 |
57.7 ± 3.3 |
67.3 ± 3.8 † |
9.52 ± 1.9 |
Tiredness after this test (%) |
53.2 ± 4.9 |
41.1 ± 3.7 |
− 12.1 ± 2.7 |
36.2 ± 3.8 |
37.6 ± 4.4 |
1.37 ± 3.1 |
Chronic fatigue syndrome score |
13.6 ± 1.3 |
11.3 ± 0.4 |
− 2.22 ± 1.4 |
12.8 ± 0.8 |
12.0 ± 0.9 |
− 0.78 ± 0.6 |
Subjects without an exercise habit |
|
|
|
|
|
|
Tiredness without exercise (%) |
34.8 ± 4.6 |
36.3 ± 3.9 |
1.52 ± 4.8 |
37.1 ± 4.7 |
27.3 ± 4.6 |
− 9.87 ± 4.7 |
Grip strength (R) (kg) |
38.7 ± 2.5 |
38.8 ± 2.6 |
0.11 ± 0.7 |
37.9 ± 2.3 |
40.0 ± 2.6 |
2.15 ± 1.0 |
Grip strength (L) (kg) |
35.5 ± 2.3 |
35.9 ± 2.5 |
0.40 ± 0.5 |
34.5 ± 2.0 |
37.6 ± 2.1 † |
3.05 ± 0.9 |
30-second chair stand test (sec) |
22.5 ± 1.3 |
24.2 ± 1.6 † |
1.67 ± 0.5 |
19.6 ± 1.5 |
25.5 ± 1.8 † |
5.90 ± 1.8 |
5-m tandem walking test (sec) |
13.2 ± 0.9 |
12.6 ± 1.1 |
− 0.60 ± 0.5 |
15.9 ± 1.4 |
11.9 ± 1.1 |
− 4.06 ± 1.6 |
Cycle ergometer test (kcal) |
38.1 ± 3.5 |
38.6 ± 3.0 |
0.44 ± 1.4 |
36.3 ± 3.5 |
44.2 ± 4.3†† |
7.95 ± 1.9 * |
Tiredness after this test (%) |
57.8 ± 4.3 |
56.9 ± 5.2 |
− 0.91 ± 4.9 |
62.5 ± 5.3 |
52.3 ± 3.2 |
− 10.2 ± 4.7 |
Chronic fatigue syndrome score |
17.6 ± 1.1 |
15.1 ± 1.3 |
− 2.47 ± 1.0 |
17.5 ± 1.4 |
13.5 ± 1.3 |
− 3.93 ± 1.4 |
Data were presented as the mean ± S.E (subjects with an exercise habit: n = 9, subjects without an exercise habit: n = 15).
Significant differences from the placebo were indicated as *: P<0.05, and those from the baseline were indicated as †: P<0.05, ††: P<0.01 (paired t-test).
Net changes in the grip strength of left hand (+2.8 vs +0.0 kg, P < 0.05), 30-second chair stand test (+6.3 vs +1.7 times, P < 0.05), 5-m tandem walking test (-3.2 vs -0.9 sec, P < 0.01), and cycle ergometer test (+8.5 vs +1.1 kcal, P < 0.01) were significantly greater after the intake of KPE than the values after the placebo ingestion. These results indicate that KPE possesses the ability to enhance physical fitness, namely, grip strength, leg muscle strength, balance, endurance, and locomotor activity (Table 3).
Physical fitness and endurance (subjects with or without an exercise habit)
In order to evaluate the influence of an exercise habit, data were recalculated by a differential analysis with or without an exercise habit. An exercise habit was defined as exercise performed once or more a week. In subjects with an exercise habit (Table 4, upper part), the grip strength of the right hand (+2.2 kg), 30-second chair stand test (+6.9 times), 5-m tandem walking test (-1.7 sec), and cycle ergometer test (+9.5 kcal) were significantly improved by KPE (P < 0.05). However, these improvements were not significantly different from those observed in placebo group.
In subjects without an exercise habit (Table 4, lower part), the grip strength of left hand (+3.1 kg, P < 0.05), 30-second chair stand test (+5.9 times, P < 0.05), and cycle ergometer test (+7.9 kcal, P < 0.01) were significantly improved by the ingestion of KPE. On the other hand, only performance in the 30-second chair stand test was significantly improved (+1.67 times, P < 0.05) after the intake of the placebo than before its ingestion. A significant difference (P < 0.05) was observed in the net change in cycle ergometer test between the placebo group and the KPE group (7.95 vs 0.44 kcal), and this was not detected in subjects without an exercise habit. However, in a differential analysis with or without an exercise habit, the average reductions observed in subjects with and without an exercise habit were not significant for any parameter tested. Therefore, these results suggest that an exercise habit is not a key factor for KPE-induced enhancements in physical fitness.
Fatigue in conventional and post-exercise states
Daily and post PFT VAS fatigue scores and CFS score were measured to evaluate the effects of KPE on fatigue. In all subjects, the daily VAS fatigue score at the conventional state was slightly decreased by ingestion of KPE or the placebo for 4 weeks (mean reduction: KPE -5.14% vs placebo -2.57%). These results were similar to the post PFT VAS fatigue scores (mean reduction: KPE -5.87% vs placebo -5.08%) and CFS scores (mean reduction: KPE -2.75 vs placebo -2.38) (Table 3).
Data for subjects without an exercise habit were re-analyzed. The results obtained showed that mean reductions after ingestion of KPE in the daily VAS fatigue score (-9.87 vs +1.52%), post PFT VAS fatigue score (-10.2 vs -0.91%), and CFS score (-3.93 vs -2.47) were greater than the placebo group (Table 4, lower part). Moreover, in the mean reductions observed in the CFS score, the treatment with KPE led to slightly better scores in subjects without an exercise habit than in those with an exercise habit (-3.93 vs -0.78, P=0.10). However, this was not the case in those receiving the placebo (-2.47 vs -2.22, P=0.93). Therefore, these results suggest that KPE improves fatigue in subjects without an exercise habit only.
Blood parameters
Blood parameters were analyzed before and after the ingestion of the test samples. Statistical evaluations were performed on 22 subjects because blood samples were not collected from two subjects (Tables 5 and 6). The results obtained showed that ingestion of KPE significantly increased total protein (+0.15 g/dL), gGTP (+3.14 U/L), number of leukocytes (+6.95×102 cells/mL), and hematocrit (+0.83%) compared to the values before ingestion, while the A/G ratio (-0.09), MCH (-0.22 pg), and MCHC (-0.40%) were lower compared to the values before the ingestion. On the other hand, ingestion of the placebo significantly increased ALT (+2.91 U/L), Cl (+0.64 mEq/L), and ketone body (+9.73 mmol/L) levels and decreased MCH (-0.27 pg) and HbA1c (-0.11%). Significant changes in total protein levels, hematocrit, and HbA1c were observed in the values after ingestion of placebo and KPE. However, these blood parameter changes were within normal ranges.
Discussion
In the present study, PFT (hand grip strength test, 30-second chair stand test, 5-m tandem walking test, and cycle ergometer test), a questionnaire about daily and post-exercise fatigue, and blood tests were performed to investigate the safety and effectiveness of KPE on physical fitness and fatigue. The results of the blood test showed that KPE did not induce abnormal changes in blood parameters compared with those by the placebo, and the significant changes observed were within normal ranges (Tables 5 and 6). Therefore, the safety of KPE was clarified in this trial condition.
Table 5. Effects of KPE on blood parameters (part 1)
Measured
parameters |
|
Unit |
Normal range |
Placebo |
KPE |
| |
|
Before |
After |
Net change (D) |
Before |
After |
Net change (D) |
Total bilirubin |
mg/dL |
0.2 - 1.2 |
0.60 |
± |
0.05 |
0.65 |
± |
0.06 |
0.05 |
± |
0.05 |
0.65 |
± |
0.06 |
0.60 |
± |
0.05 |
- 0.05 |
± |
0.05 |
Total protein |
g/dL |
6.5 - 8.3 |
7.91 |
± |
0.14 |
7.86 |
± |
0.13 |
- 0.05 |
± |
0.06 |
7.72 |
± |
0.10 |
7.87 |
± |
0.10 †† |
0.15 |
± |
0.05 * |
Albumin |
g /dL |
3.8 - 5.3 |
5.00 |
± |
0.09 |
4.96 |
± |
0.10 |
- 0.03 |
± |
0.04 |
4.97 |
± |
0.08 |
4.97 |
± |
0.08 |
0.00 |
± |
0.04 |
A/G ratio |
|
1.1 - 2.3 |
1.76 |
± |
0.06 |
1.75 |
± |
0.07 |
0.00 |
± |
0.03 |
1.83 |
± |
0.06 |
1.74 |
± |
0.06 † |
- 0.09 |
± |
0.03 |
AST |
U/L |
8 - 38 |
20.8 |
± |
0.99 |
21.8 |
± |
1.19 |
1.05 |
± |
0.64 |
21.2 |
± |
1.13 |
21.0 |
± |
0.96 |
- 0.23 |
± |
0.81 |
ALT |
U/L |
4 - 43 |
19.1 |
± |
1.70 |
22.1 |
± |
2.48 † |
2.91 |
± |
1.22 |
20.7 |
± |
2.95 |
21.6 |
± |
1.83 |
0.91 |
± |
2.42 |
ALP |
U/L |
110 - 354 |
214 |
± |
10.3 |
209 |
± |
10.5 |
- 5.73 |
± |
4.35 |
210 |
± |
11.5 |
208 |
± |
8.86 |
- 2.00 |
± |
6.61 |
LDH |
U/L |
121 - 245 |
181 |
± |
5.77 |
185 |
± |
7.53 |
3.68 |
± |
4.24 |
181 |
± |
6.18 |
182 |
± |
6.45 |
1.18 |
± |
2.85 |
gGTP |
U/L |
0 - 86 |
24.4 |
± |
2.64 |
25.4 |
± |
3.19 |
1.00 |
± |
1.08 |
22.6 |
± |
2.14 |
25.8 |
± |
2.66 † |
3.14 |
± |
1.11 |
CPK |
U/L |
38 - 196 |
156 |
± |
19.1 |
166 |
± |
18.5 |
11.2 |
± |
11.8 |
150 |
± |
15.3 |
165 |
± |
21.6 |
14.3 |
± |
13.9 |
LDL-cholesterol |
mg/dL |
70 - 139 |
120 |
± |
5.93 |
121 |
± |
6.55 |
1.68 |
± |
3.03 |
117 |
± |
6.27 |
122 |
± |
5.23 |
5.45 |
± |
2.83 |
Total cholesterol |
mg/dL |
130 - 219 |
209 |
± |
7.23 |
209 |
± |
7.60 |
- 0.27 |
± |
3.73 |
205 |
± |
7.70 |
211 |
± |
6.41 |
5.82 |
± |
3.87 |
Triglyceride |
mg/dL |
30 - 149 |
116 |
± |
14.3 |
111 |
± |
11.7 |
- 5.14 |
± |
10.2 |
119 |
± |
17.3 |
116 |
± |
13.2 |
- 3.18 |
± |
17.6 |
Phospholipids |
mg/dL |
150 - 260 |
233 |
± |
7.98 |
235 |
± |
7.68 |
1.73 |
± |
4.54 |
231 |
± |
8.95 |
239 |
± |
7.09 |
8.05 |
± |
4.20 |
Free fatty acids |
mEq/L |
0.13 - 0.77 |
0.45 |
± |
0.05 |
0.44 |
± |
0.05 |
0.00 |
± |
0.07 |
0.48 |
± |
0.06 |
0.46 |
± |
0.07 |
- 0.03 |
± |
0.06 |
HDL-cholesterol |
mg/dL |
40 - 77 |
68.4 |
± |
3.65 |
66.0 |
± |
2.90 |
- 2.45 |
± |
1.81 |
67.8 |
± |
3.32 |
67.6 |
± |
3.16 |
- 0.18 |
± |
1.43 |
Data were presented as the mean ± S.E (n = 22).
Significant differences from the placebo were indicated as *: P<0.05, and those from the baseline were indicated as †: P<0.05, ††: P<0.01 (paired t-test).
Table 6. Effects of KPE on blood parameters (part 2)
Measured |
Unit |
Normal range |
Placebo |
KPE |
parameters |
|
|
|
|
Before |
After |
Net change (D) |
Before |
After |
Net change (D) |
Na |
mEq/L |
135 - 150 |
143 |
± |
0.45 |
143 |
± |
0.37 |
0.00 |
± |
0.33 |
143 |
± |
0.35 |
142 |
± |
0.38 |
- 0.23 |
± |
0.32 |
Cl |
mEq/L |
98 - 110 |
102 |
± |
0.39 |
103 |
± |
0.32 † |
0.64 |
± |
0.29 |
103 |
± |
0.50 |
102 |
± |
0.44 |
- 0.18 |
± |
0.35 |
K |
mEq/L |
3.5 - 5.3 |
4.12 |
± |
0.08 |
4.16 |
± |
0.06 |
0.04 |
± |
0.06 |
4.11 |
± |
0.05 |
4.19 |
± |
0.04 |
0.08 |
± |
0.05 |
Urea nitrogen |
mg/dL |
8.0 - 22.0 |
13.2 |
± |
0.82 |
13.2 |
± |
0.89 |
0.05 |
± |
0.55 |
13.2 |
± |
0.72 |
13.6 |
± |
0.86 |
0.40 |
± |
0.66 |
Creatinine |
mg/dL |
0.61 - 1.04 |
0.74 |
± |
0.04 |
0.74 |
± |
0.04 |
0.00 |
± |
0.02 |
0.73 |
± |
0.03 |
0.72 |
± |
0.03 |
0.00 |
± |
0.01 |
Uric acid |
mg/dL |
3.6 - 7.0 |
5.10 |
± |
0.28 |
5.21 |
± |
0.26 |
0.10 |
± |
0.12 |
5.15 |
± |
0.28 |
5.18 |
± |
0.28 |
0.03 |
± |
0.10 |
Glucose |
mg/dL |
60 - 109 |
88.6 |
± |
3.36 |
87.7 |
± |
3.16 |
- 0.86 |
± |
3.14 |
85.5 |
± |
3.60 |
81.9 |
± |
3.59 |
- 3.59 |
± |
2.17 |
Ketone bodies |
μmol/L |
0 - 74 |
25.2 |
± |
3.13 |
34.9 |
± |
3.20 † |
9.73 |
± |
3.72 |
29.8 |
± |
2.89 |
32.8 |
± |
3.14 |
2.95 |
± |
3.59 |
Leukocyte |
× 102/μL |
39 - 98 |
80.6 |
± |
4.10 |
75.8 |
± |
4.27 |
- 4.77 |
± |
4.47 |
73.6 |
± |
3.51 |
80.5 |
± |
3.74 † |
6.95 |
± |
2.63 |
Red blood cell |
× 102/μL |
427 - 570 |
493 |
± |
8.82 |
492 |
± |
9.46 |
- 0.32 |
± |
3.73 |
490 |
± |
9.42 |
497 |
± |
9.24 |
6.77 |
± |
3.29 |
Hemoglobin |
g/dL |
13.5 - 17.6 |
15.0 |
± |
0.33 |
14.8 |
± |
0.36 |
- 0.15 |
± |
0.13 |
14.9 |
± |
0.32 |
15.0 |
± |
0.32 |
0.09 |
± |
0.11 |
Hematocrit |
% |
39.8 - 51.8 |
45.9 |
± |
0.93 |
45.6 |
± |
0.96 |
- 0.26 |
± |
0.31 |
45.3 |
± |
0.90 |
46.2 |
± |
0.95 † |
0.83 |
± |
0.36 * |
MCV |
fL |
82.7 - 101.6 |
93.1 |
± |
0.76 |
92.6 |
± |
0.78 |
- 0.44 |
± |
0.43 |
92.5 |
± |
0.76 |
92.9 |
± |
0.80 |
0.39 |
± |
0.30 |
MCH |
pg |
28.0 - 34.6 |
30.4 |
± |
0.29 |
30.1 |
± |
0.31 †† |
- 0.27 |
± |
0.06 |
30.4 |
± |
0.25 |
30.2 |
± |
0.29 † |
- 0.22 |
± |
0.09 |
MCHC |
% |
31.6 - 36.6 |
32.7 |
± |
0.22 |
32.5 |
± |
0.19 |
- 0.15 |
± |
0.16 |
32.9 |
± |
0.21 |
32.5 |
± |
0.23 †† |
- 0.40 |
± |
0.13 |
Platelet |
× 104/μL |
13.1 - 36.2 |
27.7 |
± |
1.25 |
28.3 |
± |
1.41 |
0.62 |
± |
0.59 |
27.7 |
± |
1.13 |
28.7 |
± |
1.32 |
0.97 |
± |
0.55 |
HbA1c NGSP |
% |
4.6 - 6.2 |
5.35 |
± |
0.06 |
5.24 |
± |
0.05 †† |
- 0.11 |
± |
0.02 |
5.29 |
± |
0.05 |
5.28 |
± |
0.05 |
- 0.01 |
± |
0.03 * |
Cortisol |
μg/dL |
6.2 - 19.4 |
12.8 |
± |
1.42 |
13.03 |
± |
1.04 |
0.20 |
± |
1.05 |
11.8 |
± |
1.06 |
11.7 |
± |
0.87 |
- 0.10 |
± |
0.65 |
2021 Copyright OAT. All rights reserv
Data were presented as the mean ± S.E (n = 22).
Significant differences from the placebo were indicated as *: P<0.05, and those from the baseline were indicated as †: P<0.05, ††: P<0.01 (paired t-test).
Among blood markers related to exercise in the placebo group, ALT (+2.91 U/L) and ketone body levels (+9.73 mmol/L), which increase during skeletal muscle injury [39] or after exercise [40], were significantly increased in the placebo group, but not in the KPE group (+0.91 U/L and +2.95 mmol/L). Therefore, KPE was suggested to decrease the physical load of exercise in order to maintain muscle condition.
In PFT, net changes in the grip strength of left hand (+2.8 vs +0.0 kg, P < 0.05), 30-second chair stand test (+6.3 vs +1.7 times, P < 0.05), 5-m tandem walking test (-3.2 vs -0.9 sec, P < 0.01), and cycle ergometer test (+8.5 vs +1.1 kcal, P < 0.01) were significantly better following the intake of KPE than that of the placebo (Table 1). Therefore, KPE was found to enhance physical fitness, namely, grip strength, leg strength, balance, endurance, and locomotor activity (Table 3).
On the other hand, KPE did not significantly change VAS fatigue scores in daily and post PFT and CFS scores compared with the placebo (Table 3). However, in subjects without an exercise habit, KPE slightly improved these fatigue scores compared to the values in the placebo group following a 4-week ingestion period (Table 4, lower part). Muscular mass and metabolism may be reduced in the skeletal muscle of subjects without an exercise habit. KPE was suggested to improve these decreases. We previously confirmed that PMFs, particularly 5,7-dimethoxyflavone in KPE, improved metabolism in muscle cells by activating AMPK [24]. AMPK is known to play a critical role in the regulation of energy homeostasis and is related to physical fitness, endurance, and fatigue [41-48]. For example, 5-aminoimidazole-4-carboxyamide ribonucleotide (AICAR), an AMPK agonist, was reported to increase running endurance by 44% and decrease body fat in mice following its oral administration for 4 weeks [48]. Therefore, the activation of AMPK by KPE may contribute to these activities including improvements in physical fitness performance.
Aging has also been reported to deteriorate muscular mass and metabolism [1-5]. A clinical report demonstrated that KPE effectively increased physical fitness in elderly subjects aged 60 or more [37]. Therefore, KPE may have more prominent effects on physical fitness and fatigue in elderly people and individuals lacking an exercise habit, among whom muscular metabolism has declined. A larger scale clinical study of KPE in elderly subjects or subjects without an exercise habit needs to be performed to clarify these hypotheses. In the future, KPE rich in PMFs may be utilized to enhance fitness performance and prevent locomotive dysfunctions.
Conclusion
We herein demonstrated that a 4-week treatment with KPE rich in PMFs enhanced performance in the hand grip test, 30-second chair stand test, 5-m tandem walking test, and cycle ergometer test. Furthermore, KPE intake slightly improved fatigue at the conventional state and post PFT state as well as CFS scores in subjects without an exercise habit. Therefore, KPE has the ability to enhance physical fitness, namely, grip strength, leg strength, balance, endurance, and locomotor activity. In the future, KPE rich in PMFs will be utilized to enhance fitness performance and prevent locomotive dysfunctions.
Conflicts of Interest
All authors related to this study are employees of Oryza Oil & Fat Chemical Co., Ltd. (Aichi, Japan). The authors declare no conflict of interest associated with this manuscript.
Figure / Tables and captions
Figure 1. Schedule for the study
Table 1. Subject backgrounds
Table 2. Questionnaire for CFS
Table 3. Effects of KPE on physical fitness and fatigue (part 1)
Table 4. Effects of KPE on physical fitness and fatigue (part 2)
Table 5. Effects of KPE on blood parameters (part 1)
Table 6. Effects of KPE on blood parameters (part 2)
References
- Nair KS (2005) Aging muscle. Am J Clin Nutr 81: 953-963. [Crossref]
- Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C (2006) Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev 5: 179-195. [Crossref]
- Dillon LM, Rebelo AP, Moraes CT (2012) The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life 64: 231-241. [Crossref]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, et al. (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140-1142. [Crossref]
- Miljkovic N, Lim JY, Miljkovic I, Frontera WR4 (2015) Aging of skeletal muscle fibers. Ann Rehabil Med 39: 155-162. [Crossref]
- Booth FW, Roberts CK, Laye MJ (2012) Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143-1211. [Crossref]
- Phu S, Boersma D2, Duque G3 (2015) Exercise and Sarcopenia. J Clin Densitom 18: 488-492. [Crossref]
- Phillips SM (2015) Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr 6: 452-460. [Crossref]
- Konopka AR, Harber MP (2014) Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev 42: 53-61. [Crossref]
- Fujita S, Volpi E (2006) Amino acids and muscle loss with aging. J Nutr 136: 277S-80S. [Crossref]
- Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20: 591-598. [Crossref]
- Tian G, Sawashita J, Kubo H, Nishio SY, Hashimoto S, et al. (2014) Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid Redox Signal 20: 2606-2620. [Crossref]
- Alf D, Schmidt ME, Siebrecht SC (2013) Ubiquinol supplementation enhances peak power production in trained athletes: a double-blind, placebo controlled study. J Int Soc Sports Nutr 10. [Crossref]
- Bloomer RJ, Canale RE, McCarthy CG, Farney TM (2012) Impact of oral ubiquinol on blood oxidative stress and exercise performance. Oxid Med Cell Longev 2012: 465020. [Crossref]
- Delaney CL, Spark JI, Thomas J, Wong YT, Chan LT, et al. (2013) A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 229: 1-9. [Crossref]
- Brass EP, Koster D, Hiatt WR, Amato A (2013) A systematic review and meta-analysis of propionyl-L-carnitine effects on exercise performance in patients with claudication. Vasc Med 18: 3-12. [Crossref]
- Malaguti M, Angeloni C, Hrelia S (2013) Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxid Med Cell Longev 2013: 825928. [Crossref]
- Horigome S, Yoshida I, Ito S, Inohana S, Fushimi K, et al. (2015) Inhibitory effects of Kaempferia parviflora extract on monocyte adhesion and cellular reactive oxygen species production in human umbilical vein endothelial cells. Eur J Nutr, in press. [Crossref]
- Seito LN, Sforcin JM, Bastos JK, Di Stasi LC (2015) Zeyheria montana Mart. (Bignoniaceae) as source of antioxidant and immunomodulatory compounds with beneficial effects on intestinal inflammation. J Pharm Pharmacol 67: 597-604. [Crossref]
- Horigome S, Yoshida I, Tsuda A, Harada T, Yamaguchi A, et al. (2014) Identification and evaluation of anti-inflammatory compounds from Kaempferia parviflora. Biosci Biotechnol Biochem 78: 851-860. [Crossref]
- Panthong A, Tassaneeyakul W, Kanjanapothi D, Tantiwachwuttikul P, Reutrakul V (1989) Anti-inflammatory activity of 5,7-dimethoxyflavone. Planta Med 55: 133-136. [Crossref]
- Kim M, Kim N, Han J (2014) Metabolism of Kaempferia parviflora polymethoxyflavones by human intestinal bacterium Bautia sp. MRG-PMF1. J Agric Food Chem 62: 12377-12383. [Crossref]
- Tsuji PA, Winn RN, Walle T (2006) Accumulation and metabolism of the anticancer flavonoid 5,7-dimethoxyflavone compared to its unmethylated analog chrysin in the Atlantic killifish. Chem Biol Interact 164: 85-92. [Crossref]
- Toda K, Takeda S, Hitoe S, Nakamura S, Matsuda H, et al. (2016) Enhancement of energy production by black ginger extract containing polymethoxy flavonoids in myocytes through improving glucose, lactic acid and lipid metabolism. J Nat Med 70: 163-172. [Crossref]
- Collaert B, Wijnen L, De Bruyn H (2011) A 2-year prospective study on immediate loading with fluoride-modified implants in the edentulous mandible. Clin Oral Implants Res 22: 1111-1116. [Crossref]
- Temkitthawon P, Hinds TR, Beavo JA, Viyoch J, Suwanborirux K, et al. (2011) Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J Ethnopharmacol 11: 1437-1441. [Crossref]
- Tep-Areenan P, Sawasdee P, Randall M (2010) Possible mechanisms of vasorelaxation for 5,7-dimethoxyflavone from Kaempferia parviflora in the rat aorta. Phytother Res 24: 1520-1525. [Crossref]
- Sookkongwaree K, Geitmann M, Roengsumran S, Petsom A, Danielson UH (2006) Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Pharmazie 61: 717-721. [Crossref]
- Akase T, Shimada T, Terabayashi S, Ikeya Y, Sanada H, et al. (2011) Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J Nat Med 65: 73-80. [Crossref]
- Shimada T, Horikawa T, Ikeya Y, Matsuo H, Kinoshita K, et al. (2011) Preventive effect of Kaempferia parviflora ethyl acetate extract and its major components polymethoxyflavonoid on metabolic diseases. Fitoterapia 82: 1272–1278. [Crossref]
- Rujjanawate C, Kanjanapothi D, Amornlerdpison D, Pojanagaroon S (2005) Anti-gastric ulcer effect of Kaempferia parviflora. J Ethnopharmacol 102: 120-122. [Crossref]
- Kusirisin W, Srichairatanakool S, Lerttrakarnnon P, Lailerd N, Suttajit M, et al. (2009) Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med Chem 5: 139–147. [Crossref]
- Chaturapanich G, Chaiyakul S, Verawatnapakul V, Yimlamai T, Pholpramool C (2012) Enhancement of aphrodisiac activity in male rats by ethanol extract of Kaempferia parviflora and exercise training. Andrologia 44 Suppl 1: 323-328. [Crossref]
- Ninomiya K, Matsumoto T, Chaipech S, Miyake S, Katsuyama Y, et al. (2016) Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora. J Nat Med 70: 179-189. [Crossref]
- Yorsin S, Kanokwiroon K, Radenahmad N, Jansakul C (2014) Effects of Kaempferia parviflora rhizomes dichloromethane extract on vascular functions in middle-aged male rat. J Ethnopharmacol 156: 162-174. [Crossref]
- Promthep K, Eungpinichpong W, Sripanidkulchai B, Chatchawan U (2015) Effect of Kaempferia parviflora extract on physical fitness of soccer players: a randomized double-blind placebo-controlled trial. Med Sci Monit Basic Res 21: 100-108. [Crossref]
- Wattanathorn J, Muchimapura S, Tong-Un T, Saenghong N, Thukhum-Mee W, et al. (2012) Positive modulation effect of 8-week consumption of Kaempferia parviflora on health-related physical fitness and oxidative status in healthy elderly volunteers. Evid Based Complement Alternat Med 732816.
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, et al. (1993) Development of a fatigue scale. J Psychosom Res 37: 147-153. [Crossref]
- Nathwani RA, Pais S, Reynolds TB, Kaplowitz N (2005) Serum alanine aminotransferase in skeletal muscle diseases. Hepatology 41: 380-382. [Crossref]
- Matoulek M, Svobodova S, Vetrovska R, Stranska Z, Svacina S (2014) Post-exercise changes of beta hydroxybutyrate as a predictor of weight changes. Physiol Res 63 Suppl 2: S321-325. [Crossref]
- Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15-25. [Crossref]
- Hardie DG, Schaffer BE, Brunet A (2016) AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol 26: 190-201. [Crossref]
- Astratenkova IV, Rogozkin VA (2013) [Participation AMPK in the regulation of skeletal muscles metabolism]. Ross Fiziol Zh Im I M Sechenova 99: 657-673. [Crossref]
- Baltgalvis KA, White K, Li W, Claypool MD, Lang W, et al. (2014) Exercise performance and peripheral vascular insufficiency improve with AMPK activation in high-fat diet-fed mice. Am J Physiol Heart Circ Physiol 306: 1128-1145. [Crossref]
- Hardie DG (2004) AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc 36: 28-34. [Crossref]
- Myburgh KH (2004) Protecting muscle ATP: positive roles for peripheral defense mechanisms-introduction. Med Sci Sports Exerc 36: 16-19. [Crossref]
- Lantier L, Fentz J, Mounier R, Leclerc J, Treebak JT, et al. (2014) AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J 28: 3211-3224. [Crossref]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, et al. (2008) AMPK and PPARdelta agonists are exercise mimetics. Cell 134: 405-415. [Crossref]